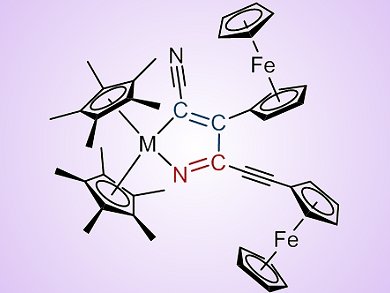
The formation and reactivity of small metallacycles, such as metallacyclopropenes, is well known; however, the formation of the analogous heterometallacycles, by the heterocoupling of a nitrile and an alkyne or the homocoupling of two nitriles, has received little attention.
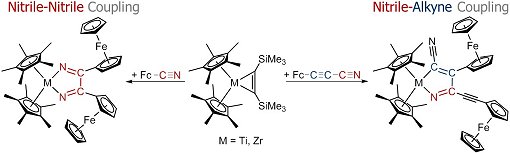
Heinrich Lang, Technische Universität Chemnitz, Germany, and Uwe Rosenthal, Universität Rostock, Germany, and co-workers have discovered that the treatment of Group 4 (Ti and Zr) metallocene alkyne complexes with ferrocenyl-substituted nitriles Fc–C≡N and Fc–C≡C–C≡N results in nitrile–nitrile C–C homocoupling and nitrile–alkyne heterocoupling, respectively. Both reactions proved to be high yielding and highly selective. Indeed, for Fc–C≡N, the application of η5-pentamethylcyclopentadienyl (Cp*) ligands resulted in the coupling of the two nitriles by means of C–C bond formation, rather than by C–N or N–N bond formation, to give 1-metalla-2,5-diaza-cyclopenta-2,4-dienes. Interestingly, 3-ferrocenyl-2-propyne-nitrile (Fc–C≡C–C≡N) reacted differently, resulting in nitrile–alkyne C–C coupling of the two nitriles and the synthesis of 1-metalla-2-aza-cyclopenta-2,4-dienes.
Electrochemical measurements were also carried out by using cyclic voltammetry and these show irreversible oxidations, which initiate decomposition, for all of the heterometallacycles synthesized. For the 1-metalla-2,5-diaza-cyclopenta-2,4-dienes the influence of the ferrocenyl substituent is particularly interesting. The ferrocenyl–zirconium compound was found to be more stable against oxidation than the analogous tolyl–zirconium compound, whereas for the titanium complexes the stability was reversed. For the 1-metalla-2-aza-cyclopenta-2,4-dienes, redox-active products and several redox processes were observed. Owing to the different potentials of the reaction intermediates, different decomposition mechanisms have been predicted.
- Unusual Nitrile–Nitrile and Nitrile–Alkyne Coupling of Fc–C≡N and Fc–C≡C–C≡N,
Lisanne Becker, Frank Strehler, Marcus Korb, Perdita Arndt, Anke Spannenberg, Wolfgang Baumann, Heinrich Lang, Uwe Rosenthal,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304478