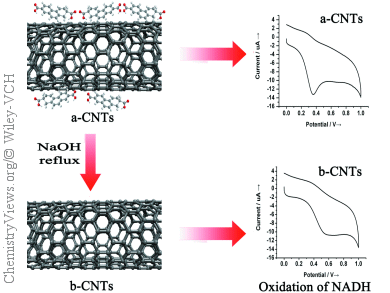
Carbon nanotubes (CNTs) are widely employed as electrode materials and are claimed to display dramatically improved electrochemical behavior compared to conventional carbon materials. However, recently it has been demonstrated that the electrocatalysis of CNTs might be due to the presence of some impurities, such as metallic catalysts, nanographitic particles, and amorphous carbon. For this reason, CNTs are usually purified or treated with nitric acid or nitric and sulphuric acid prior to their versatile applications. Unfortunately, these strongly acidic and oxidative conditions lead to erosion of the CNT structures, which creates defects on the sidewalls and gives rise to numerous molecular byproducts, commonly referred as carboxylated carbonaceous fragments (CCFs).
Liande Zhu and co-workers, Northeast Normal University (NENU), Changchun, China, studied the impact that these CCFs may be having on the electrochemical properties of CNTs. The group chose ascorbic (AA) and β-nicotinamide adenine dinucleotide (NADH) for the electrochemical probes as their electrode kinetics are insensitive to metallic impurities, such as Fe, Co, Mo, and Ni, and tested nitric acid purified CNTs for influence of CCF contamination.
Interestingly, they found that the electrocatalytic activities of the CNTs are actually dominated by the adsorbed CCFs generated during the acidic pretreatment. After removal of the CCFs by base rinse, the electrocatalytic properties of CNTs are greatly deteriorated and degraded to a level similar to the conventional graphite powder.
- The Significant Role of Carboxylated Carbonaceous Fragments in the Electrochemistry of Carbon Nanotubes,
Xiao Ma, Li Jia, Lu Zhang, Liande Zhu,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304311