James Mason Crafts was born on March 8, 1839, in Boston, USA. As a young boy he showed promise in the physical sciences, including chemistry. He also had the good fortune to personally know William Barton Rogers, who went on to found the Massachusetts Institute of Technology (MIT), Cambridge, USA.
After obtaining a Bachelor of Science in the USA, he moved to Germany initially to study mining but he then became interested in chemistry. He worked as a research assistant in chemistry, first in Germany and then in Paris, where he met Charles Friedel, with whom he became good friends and worked closely with. In 1865 he returned to the USA and in 1868, he became the first professor of chemistry at Cornell University, Ithaca. After five years, he was appointed as professor of chemistry at MIT where he remained until 1874, when owing to bad health he had to take a break from this position. He returned to Paris, with the initial intention to stay for only one year, but he ended up staying for 17 years, working in the laboratories of Wurtz and Friedel.
His many achievements across physical and organic chemistry include inventing a new hydrogen thermometer, measuring the densities of iodine at very high temperatures, and preparing a number of new compounds of the element silicon and of arsenic.
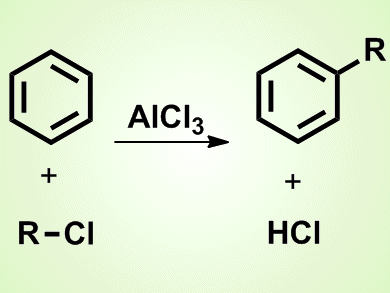
James Mason Crafts is best known for the development, jointly with Charles Friedel, of the Friedel–Crafts reactions in 1877. They were the first to report the use of a Lewis acid, that is aluminum chloride, in organic synthesis. These reactions allowed the synthesis of substitued aromatics, thus enabling the synthesis of hundreds of new organic compounds. The main types are Friedel–Crafts alkylation (see picture) and acylation.
The Friedel–Crafts acylation has a number of advantages over the alkylation. Issues with carbocation rearrangement are avoided and polyacylation does not occur as, unlike with the alkylation, the products are less reactive than the starting material.
These reactions are still commonly used to synthesize substitued aromatics.
Source
- James Mason Crafts,
Avery A. Ashdown,
J. Chem. Educ. 1928, 5 (8), 911.
https://doi.org/10.1021/ed005p911
James Mason Crafts is the answer to Guess the Chemist (27).




Desire to be like him