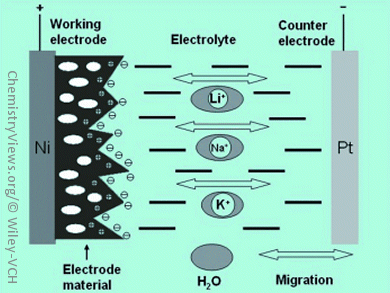
Rechargeable batteries represent one type of electrical energy-storage system (ESS) technology. They are capable of reversibly storing and releasing electrical energy without involving Carnot cycles, thus, potentially allowing for high efficiencies. Current state-of-the-art battery technologies available for large-scale applications are either too costly, e.g. sodium–sulfur and lithium-ion batteries, or unsuitable for enduring deep charge/discharge cycling over a long period of time, i.e. the lead-acid battery.
Craig Banks, Manchester Metropolitan University, UK, together with Xiaobo Ji and co-workers, Central South University, Changsha, PR China, present an aqueous sodium-ion battery with a Na3V2(PO4)3 (NVP) electrode, in which the alkaline ions of the aqueous electrolyte (1 M Na2SO4) can intercalate the electrode compound. This leads to a reversible capacity similar to that observed in aqueous lithium-ion batteries. The power of the battery is expected to be superior, because of the promising ion conductivity of NVP in addition to the high conductivity of the aqueous electrolyte. Moreover, replacement of lithium-ion electrodes and electrolytes with sodium-ion-based systems can further reduce the cost of the components assembled in aqueous batteries.
This initial exploration of NVP in aqueous electrolyte provides insights for the fabrication of a new type of sodium-ion battery, which should be safe, low cost, and environmentally friendly, as well as exhibit a high capacity.
- Aqueous Sodium-Ion Battery using a Na3V2(PO4)3 Electrode,
Weixin Song, Xiaobo Ji, Yirong Zhu, Hanjun Zhu, Fangqian Li, Jun Chen, Fang Lu, Yinpeng Yao, Craig. E. Banks,
ChemElectroChem 2014.
DOI: 10.1002/celc.201300248