Bromodomains (BRDs) are small regions found in a variety of proteins that recognize and bind acetylated histone tails. This binding affects chromatin structure and facilitates the localization of transcriptional complexes to specific genes, thereby regulating epigenetically controlled processes, such as gene transcription and mRNA elongation. Inhibitors of the bromodomain and extra-terminal (BET) proteins BRD2–4 and T, which prevent bromodomain binding to acetylated histone tails, have shown therapeutic promise in several diseases.
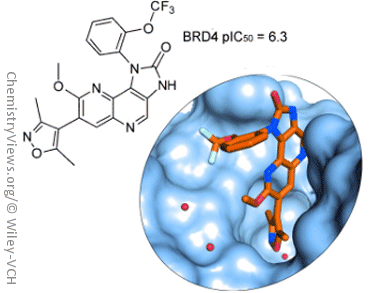
Olivier Mirguet and colleagues, GlaxoSmithKline, France and UK, report the discovery of new naphthyridine analogues as potent BET bromodomain family inhibitors. To expand the chemical diversity of BET inhibitors and enhance solubility of the parent chemical series, namely the isoxazoloquinoline derivatives, one extra nitrogen atom was added to the quinoline template.
Assessment of the best position for the nitrogen atom resulted in the synthesis of 1,5-naphthyridine compounds, which showed a higher affinity over the other isomers. To explain this higher affinity, Mirguet’s team solved X-ray crystal structures of naphthyridine isomers and carried out quantum mechanical calculations.
The most promising compounds were progressed in a mouse model of inflammation and exhibited dose-dependent anti-inflammatory pharmacology and showed efficacy.
- Naphthyridines as Novel BET Family Bromodomain Inhibitors,
Olivier Mirguet, Yann Lamotte, Chun-wa Chung, Paul Bamborough, Delphine Delannée, Anne Bouillot, Françoise Gellibert, Gael Krysa, Antonia Lewis, Jason Witherington, Pascal Huet, Yann Dudit, Lionel Trottet, Edwige Nicodeme,
ChemMedChem 2014, 9, 580–589.
DOI: 10.1002/cmdc.201300259
For more new developments on epigenetics & drug discovery see:
- Special Issue: Epigenetics & Drug Discovery,
ChemMedChem 2014, 9(3).