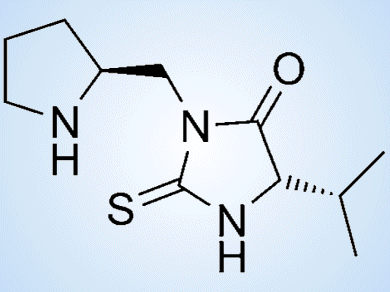
Organocatalysis has become a powerful tool in modern organic synthesis, complementing asymmetric metal-catalyzed and biocatalyzed transformations. It has led to the development of new convenient strategies to synthesize a variety of chiral building blocks.
As part of their project on the development of more sustainable organocatalytic procedures, Eusebio Juaristi and co-workers, Centro de Investigación y de Estudios Avanzados del Instituto Politénico Nacional (CINVESTAV), Mexico City, Mexico, have explored the use of mechanochemical energy under solvent-free reaction conditions. In this context, they have reported a convenient synthetic procedure for the preparation of chiral thiohydantoins (pictured) by coupling a protected L-proline derivative with isothiocyanates derived from a number of natural α-amino acids.
These chiral heterocycles were tested as organocatalysts in the asymmetric Michael addition reaction. The reactions of interest proceeded with high diastereo- and enantioselectivity, with various Michael acceptors and under solvent-free conditions.
Such reactions could provide a key method for decreasing the environmental impact of such chemical transformations.
- An Alternative Synthesis of Chiral (S)-Proline Derivatives that Contain a Thiohydantoin Moiety and Their Application as Organocatalysts in the Asymmetric Michael Addition Reaction under Solvent-Free Conditions,
Alberto Vega-Peñaloza, Omar Sánchez-Antonio, C. Gabriela Ávila-Ortiz, Margarita Escudero-Casao, Eusebio Juaristi,
Asian J. Org. Chem. 2014, 3, 487–496.
DOI: 10.1002/ajoc.201400018