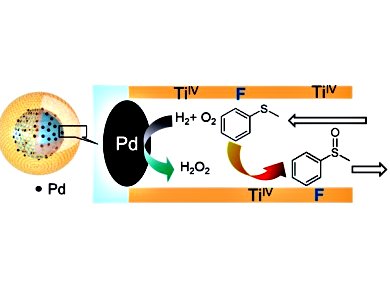
There are many advantages to using hydrogen peroxide as an oxidizing agent, including its versatility and its environmentally friendly nature. However, the unstable nature of H2O2 makes it challenging to synthesize and store. For this reason, researchers have been investigating ways to generate H2O2 in the same vessel as the desired reaction with Pd-based catalysts.
Hiromi Yamashita and colleagues, Osaka University, Japan, have developed a catalyst for this purpose composed of a Pd-nanoparticle-decorated silica core covered by a mesoporous silica shell (see figure). In this structure, H2O2 is generated at the core and then performs the desired reaction at Ti or Fe catalytic sites in the shell before it has a chance to decompose.
To improve the catalytic activity of these materials, the researchers took advantage of the difference in hydrophobicity between the reactants and products in the oxidation of methyl phenyl sulfide. After modifying the shell material with a fluorine-containing silylation reagent, the nanostructures became more hydrophobic, which enhanced the adsorption of the hydrophobic reactants. The resulting catalysts exhibited three times higher catalytic activity compared with previously reported unmodified catalysts and 20 times higher activity than traditional catalysts with zeolite supports. In addition, they also exhibited improved selectivity towards the desired sulfoxide product, and could be used for the Fe-based oxidation of cyclohexane.
- Hydrophobic Modification of Pd/SiO2@Single-Site Mesoporous Silicas by Triethoxyfluorosilane: Enhanced Catalytic Activity and Selectivity for One-Pot Oxidation,
Kazuki Nakatsuka, Kohsuke Mori, Shusuke Okada, Shohei Ikurumi, Takashi Kamegawa, Hiromi Yamashita,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402586