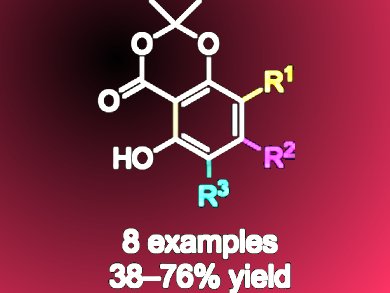
γ-Resorcylates (2,6-dihydroxybenzoates) are used in medicine, polymer science, dye synthesis, as well as as ligands for metal–organic chemistry.
Peter S. Blencowe and Anthony G. M. Barrett, Imperial College London, UK, have developed three efficient biomimetic syntheses of functionalized γ-resorcylates from 2,2,6-trimethyl-4H-1,3-dioxin-4-one derivatives.
The first step is a cross metathesis reactions of 2,2-dimethyl-6-vinyl-4H-1,3-dioxin-4-one with homoallylic esters. Or, alternatively, aldol reactions of tert-butyl or benzyl esters with 1-(2,2-dimethyl-4-oxo-4H-1,3-dioxin-6-yl)-acetone and related ketones. This provides a versatile modular approach for the synthesis of the acids below, which undergo aromatization under mild Appel-type reaction conditions to give γ-resorcylates selectively incorporating up to two alkyl and/or aryl substituents.
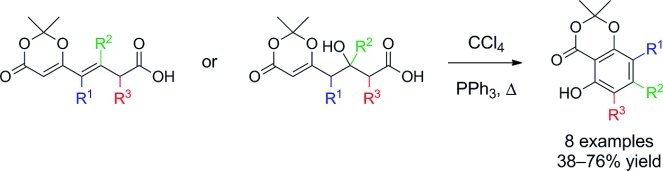
This method also provides access to 4-substituted γ-resorcylates which previously have not been readily available.
- Biomimetic Synthesis of Functionalized γ-Resorcylates from Dioxinone Derivatives,
Peter S. Blencowe, Anthony G. M. Barrett,
Europ. J. Org. Chem. 2014.
DOI: 10.1002/ejoc.201402473