2,2,5-Trisubstituted tetrahydrofurans (THF) are found in many natural products used as pharmaceuticals, biologically active natural products, and versatile building blocks in organic synthesis. However, synthesis strategies for catalytic enantioselective formation of the stereocenters at the 2- and 5-positions of the tetrahydrofuran scaffolds are rare.
Jia-Rong Chen and Wen-Jing Xiao, Central China Normal University, Wuhan, China, and colleagues have developed a sequential one-pot Cu-catalyzed asymmetric Henry reaction and iodocyclization of γ,δ-unsaturated alcohols. The reaction provided efficient access to a range of biologically and synthetically important 2,5-polysubstituted tetrahydrofuran derivatives in high yields with excellent enantioselectivities (up to 97% ee).
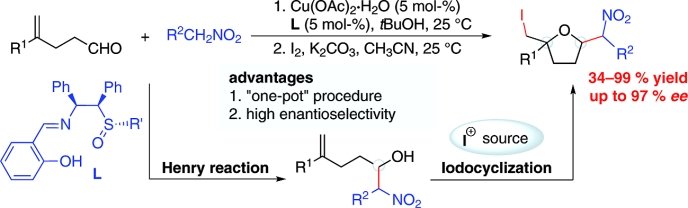
The diastereoisomers of all the products could easily be separated and the products could be transformed into the corresponding chiral amines with excellent diastereo- and enantioselectivities.
The team hopes to use these ligands for the Lewis acid catalyzed asymmetric synthesis of other valuable heterocycles.
- Enantioselective Synthesis of Tetrahydrofuran Derivatives by Sequential Henry Reaction and Iodocyclization of γ,δ-Unsaturated Alcohols,
Li-Yan Chen, Jia-Rong Chen, Hong-Gang Cheng, Liang-Qiu Lu, Wen-Jing Xiao,
Europ. J. Org. Chem. 2014.
DOI: 10.1002/ejoc.201402396




