137Cs is extremely dangerous as it has a high fission yield of 6.09 % and a half-life of 30.17 years, and its salts are highly water-soluble. 137Cs+ ions must also be effectively removed from nuclear waste in reprocessing plants, however, this task is difficult as the 137Cs+ ion concentration is lower than other cations present.
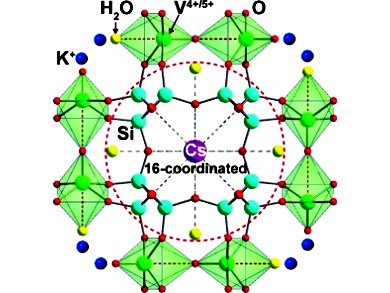
Kyung Byung Yoon and co-workers, Sogang Unversity, Seoul, South Korea, have developed a porous vanadosilicate material that contains both V4+ and V5+ ions. This material is a better Cs+ remover than the current leader in the field, which is a protonated crystalline silicotitanate. The new vanadate material forms a hexadeca-coordinated complex with the Cs+ ions, which is the largest coordination number reported to date for any metal center. The complex also contains extremely strong Cs+−O bonds.
The researchers carried out a series of test experiments for the removal of Cs+ ions from groundwater, seawater, and highly basic nuclear waste and confirmed that their new material is superior to at least five other existing materials.
- A Novel Vanadosilicate with Hexadeca-Coordinated Cs+Ions as a Highly Effective Cs+ Remover,
Shuvo Jit Datta, Won Kyung Moon, Do Young Choi, In Chul Hwang, Kyung Byung Yoon,
Angew. Chem. Int. Ed. 2014, 53, 7203–7208.
DOI: 10.1002/anie.201402778
|
The hexadeca-coordinated complex with the Cs+ ions is the largest coordination number reported to date for any metal center. |
|
 |
Find more world records from all branches of chemistry on the Records and Challenges platform of The Chemical Record, under www.tcr.wiley-vch.de/records. |