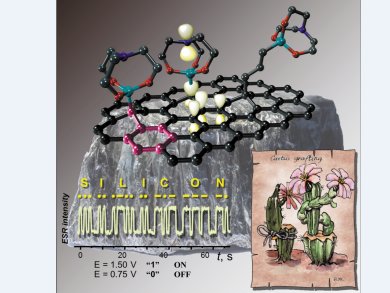
To build an “organic electrode”, an orbital-conjugated link between a carbon conductor and a molecular graft must be formed. This can be achieved by a connecting molecular entity to graphite. The σ-bond of the molecular entity should be perpendicular to the surface and hence would be involved in σ–π conjugation with the π-system of graphite.
Hyperconjugative interactions of Si–C bonds are known to be quite efficient, so Viatcheslav Jouikov and his group at the Université de Rennes 1, France, thought that redox-active orbital units with axial symmetry might be found among organosilicon derivatives, namely, in the family of metallatranes. They have developed a method to provide an easy and convenient way for building silatranyl-modified glassy carbon surfaces, in which functional groups of silatrane precursors are grafted onto the carbon surface by means of a radical-based reaction, catalyzed by sub-nanomolar amounts of transition metals.
The benzyl-type electron overlap in the silatrane–graphite system provides very low charge-transfer resistance, making it a promising model of “organic electrodes”. Fast and reproducible electrochemically driven conversion of silatranes to persistent paramagnetic states can be of interest for spin-writing, electroanalytical applications, and catalysis.
- Covalent Grafting of Silatranes to Carbon Interfaces,
Charles Peureux, Viatcheslav Jouikov,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403091