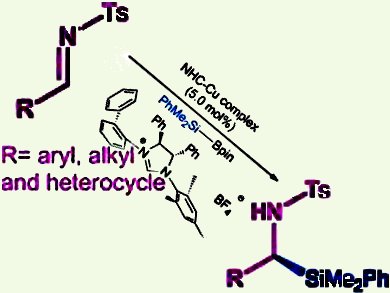
α-Silyl amines are becoming increasingly important because of the potential positive influence of the silicon atoms on their physicochemical behavior and biological performance. Racemic syntheses have been previously reported, but the enantioselective variant has remained a challenge. In this context, the asymmetric 1,2-addition of silicon nucleophiles to aldimines is a viable approach. However, the key lies in identifying a suitable chiral catalyst that can achieve facile activation of the silicon source as well as excellent asymmetric induction.
Wei He and co-workers from Tsinghua University, Beijing, China, discovered that a copper(I)/chiral N-heterocyclic carbene (NHC) is an ideal catalyst. Their method features Suginome’s reagent (Me2PhSiBpin) and an easily accessible robust NHC ligand, which is insensitive to reagent sources/moisture/air in this reaction. Notably, a wide array of aromatic and aliphatic aldimines are applicable in this reaction and furnished the desired products in 77–94 % yield and up to 99 % ee.
The enantioselective 1,2-addition to ketimines was also explored, but the low reactivity of the ketimines led to inferior results and remains a challenge.
- Enantioselective Syntheses of α-Silyl Amines via a Copper-N-Heterocyclic Carbene Catalyzed Nucleophilic Silicon Transfer to Imines,
Chunliang Zhao, Chenran Jiang, Jing Wang, Cai Wu, Qing-Wei Zhang, Wei He,
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402077