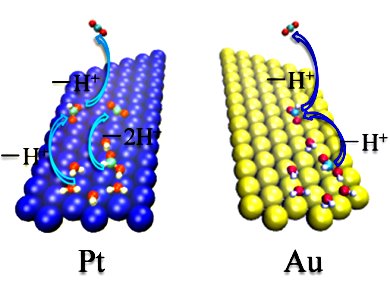
The oxidation of formic acid is important as a prototype reaction for the oxidation of small organics and as such has relevance for understanding low-temperature fuel cells. Both Pt catalysts and Au electrodes have been used extensively to study HCOOH oxidation. However, a fundamental question remains to be answered: Does HCOOH oxidation proceed through the same mechanism on Pt and Au?
Wang Gao and Timo Jacob, Universität Ulm, Germany, together with collaborators at Jilin University, China, have found that, under gas-phase conditions, the HCOOH oxidation with both Au and Pt proceeds through the same mechanism, involving a single formate as active intermediate. However, under electrochemical conditions, the mechanism for the Au and Pt methods are substantially different. On Au, the reaction proceeds via the formate pathway or a mechanism involving HCOOH–HCOO complexes, whereas on Pt it occurs through the formate and/or direct pathways depending on the electrode potential and coadsorbed CO*/OH*.
- Revealing the Active Intermediates in the Oxidation of Formic Acid on Au and Pt(111),
Wang Gao, Er Hong Song, Qing Jiang, Timo Jacob,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402737