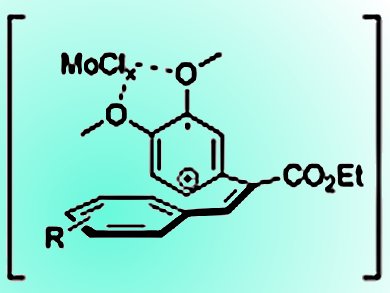
The synthesis of phenanthrene derivatives is of increasing interest owing to the diverse biological and pharmacological properties of these compounds, including antitumor and antibacterial activities. Although there are several routes to phenanthrene derivatives, many of these methods suffer from poor atom economy and a large number of linear steps.
Siegfried R. Waldvogel, Mainz University, Germany, and co-workers have developed a C–C coupling reaction of 2-aryl-substituted cinnamates (1) to form the corresponding phenanthrene derivatives (2), in yields up to 99 %, by using a MoCl5/TiCl4 reagent mixture.
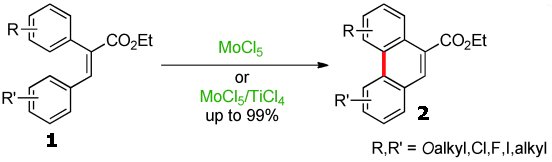
This oxidation reaction is easy to perform, and the best results are obtained when the 2-phenyl substituent of the cinnamate is equipped with two methoxy groups. Importantly, the high rate of C–C bond formation can tolerate a variety of substituents that are not compatible with other reagents.
This simple, inexpensive, but powerful method emphasizes the effectiveness of MoCl5 or MoCl5/TiCl4 in oxidative coupling reactions.
- Oxidative Cyclization Reaction of 2-Aryl-Substituted Cinnamates To Form Phenanthrene Carboxylates by Using MoCl5 ,
Kathrin Wehming, Moritz Schubert, Gregor Schnakenburg, Siegfried^^R. Waldvogel,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403442