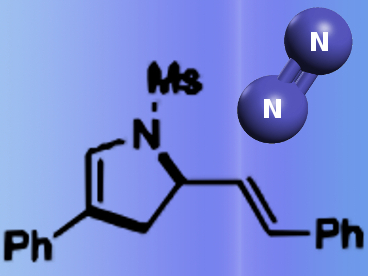
Azaheterocycles are a highly important class of compounds due to their biological activities and pharmaceutical applications. In particular, dihydroazepines, dihydropyrroles, and pyrroles are constituents of a valuable privileged structure in organic chemistry.
Phil Ho Lee and co-workers from Kangwon National University, Republic of Korea, made these compounds by an overall aza-[4+3] annulation reaction through sequential [3+2]/[2+1] cycloadditions-aza-Cope rearrangement. Aza-[3+2] annulation was achieved through [3+2]/[2+1]-aza-Cope rearrangement-1,3-migration or [3+2]/[2+1]-Clock rearrangement. This method provides a straightforward synthetic route from simple triazoles to dihydroazepines and dihydropyrroles with liberation of N2 as the single byproduct.
This procedure was successfully applied to a one-pot process starting from terminal alkynes, azides, and dienes. A wide range of substrates in terminal alkynes and 1,3-dienes are selectively annulated with excellent functional group tolerance, thus opening a new and practical avenue for the production of azaheterocycles.
- Aza-[4+3] and Aza-[3+2] Annulations for Synthesis of Dihydroazepines and Dihydropyrroles from Alkynes, Sulfonyl Azides, and 1,3-Dienes
Sanghyuck Kim, Juntae Mo, Jaeeun Kim, Taekyu Ryu, Phil Ho Lee
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402071