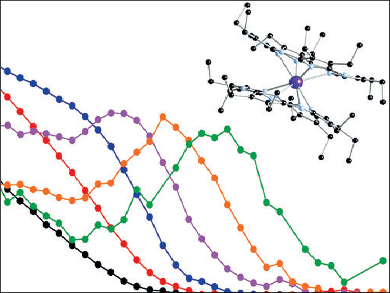
Single-molecule magnets (SMMs) have been proposed for applications in high-density information storage and for quantum computing. However, most SMMs only show magnet-like behavior at blocking temperatures (TB) near that of liquid helium. Thus, the realization of reasonably high TB is of great interest. The best SMMs remain the lanthanide-based double-decker phthalocyanine (Pc) complexes discovered by Ishikawa and colleagues [1]. These exhibit TB ≥ 55 K for the [Tb(Pc)2] derivative.
Pietro Carretta, University of Pavia, Italy, together with Pablo Ballester and José Ramón Galán-Mascarós, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues have synthesized double-decker complexes of lanthanides (Gd, Tb, Dy) with octaethyltetraazaporphyrin. The Tb3+ and Dy3+ derivatives show SMM behavior with high TB values over 50 and 10 K, respectively.
The use of tetraazaporphyrin ligands rather than the Pc counterparts gives increased chemical versatility and creates complexes that are highly soluble in nonpolar organic solvents and can be conveniently sublimed under relatively mild conditions. This easy processing opens the way for the incorporation of these single-ion magnets into nanostructures through typical procedures such as dip coating or ultra-high vacuum techniques.
- Single-Molecule-Magnet Behavior in the Family of [Ln(OETAP)2] Double-Decker Complexes
(Ln=Lanthanide, OETAP=Octa(ethyl)tetraazaporphyrin),
Nelson Giménez-Agulló, Cristina Sáenz de Pipaón, Louis Adriaenssens, Marta Filibian, Marta Martínez-Belmonte, Eduardo C. Escudero-Adán, Pietro Carretta, Pablo Ballester, José Ramón Galán-Mascarós,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402869
[1] Miki Sugita, Tadahiko Ishikawa, Shin-ya Koshihara, Youkoh Kaizu, Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level, Naoto Ishikawa, J. Am. Chem. Soc. 2003, 125, 8694–8695. DOI: 10.1021/ja029629n