Hydrogen storage is an important concept for the vision of a world in which renewable energy from the sun or wind will be used for the production of H2, which can then be stored and converted back to electricity in fuel cells on demand. Of the various hydrogen storage materials under investigation, liquid organic hydrogen carriers (LOHCs) can be conveniently dehydrogenated and hydrogenated in a reversible manner. In particular the dehydrogenation of LOHCs can be favorably combined with the chemical recycling of CO2 to yield valuable products, for example methanol or formic acid (FA).
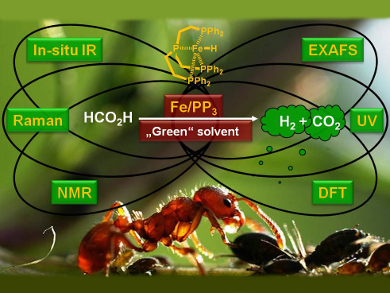
For such reactions, a current goal in organometallic catalysis is the substitution of noble metals by non-noble metals. Matthias Beller, Leibniz Institute for Catalysis, Rostock, Germany, in collaboration with groups from the Universities of Rostock and Paderborn, both Germany, and the École Polytechnique Fédérale de Lausanne, Switzerland, has developed one of the most active iron catalysts for FA dehydrogenation known to date. It was generated in situ from cationic FeII/FeIII precursors and tris[2-(diphenylphosphino)ethyl]phosphine (PP3). The optimal catalytic performance was achieved by using [FeH(PP3)]BF4/PP3 in propylene carbonate in the presence of traces of water. With the exception of fluoride, the presence of halide ions in solution inhibited the catalytic activity.
The catalyst system has comparable activity to most known noble-metal catalysts used for this transformation. Spectroscopic investigations give an insight into the reaction mechanism and an iron–formate complex was identified as the key active species.
- Base-Free Non-Noble-Metal-Catalyzed Hydrogen Generation from Formic Acid: Scope and Mechanistic Insights,
Dörthe Mellmann, Enrico Barsch, Matthias Bauer, Kathleen Grabow, Albert Boddien, Anja Kammer, Peter Sponholz, Ursula Bentrup, Ralf Jackstell, Henrik Junge, Gábor Laurenczy, Ralf Ludwig, Matthias Beller,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403602
Also of interest:
- Emil Fischer Medal 2014
Professor Matthias Beller, Leibniz-Institut für Katalyse (LIKAT), Rostock, Germany, honored