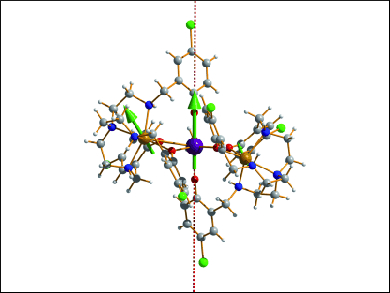
The blocking temperature of single-molecule magnets (SMMs) defines the range in which their magnetic state remains unperturbed and hence their applicability for high-density information storage and quantum processing. It has so far been a challenge to attain high blocking barriers for polynuclear SMMs based on 3d and 4f metals, as a result of magnetic interactions between the various metal sites.
The groups of Ming-Liang Tong, Sun Yat-Sen University, Guangzhou, China, Annie Powell, Karlsruhe Institute of Technology (KIT), Germany, and Liviu Ungur, KU Leuven, Belgium, formally replaced the diamagnetic ZnII ion in the single-ion magnet [ZnII2DyIII] with a paramagnetic 3d ion to obtain the new complex [FeII2DyIII]. This species exhibited a record anisotropy barrier for d-f SMMs of 319 cm−1 (459 K), which is over three times higher than the next highest 3d-nf SMM.
Structural analysis by Mössbauer spectroscopy and ab initio calculations revealed a D5h DyIII ion and two asymmetric and distorted FeII ions, and the higher blocking barrier of [FeII2DyIII] was rationalized by the enhancement of the axial crystal field due to the paramagnetic Fe ions and the altered geometry of the DyIII environment.
- A Heterometallic FeII-DyIIISingle-Molecule Magnet with a Record Anisotropy Barrier,
Jun-Liang Liu, Jie-Yi Wu, Yan-Cong Chen, Valeriu Mereacre, Annie K. Powell, Liviu Ungur, Liviu F. Chibotaru, Xiao-Ming Chen, Ming-Liang Tong,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201407799
 The highest anisotropy barrier for any d-f single-molecule magnet (319 cm−1, 459 K) was reported for an FeII–DyIII–FeII complex. Find more world records from all branches of chemistry on the Records and Challenges platform of The Chemical Record.
The highest anisotropy barrier for any d-f single-molecule magnet (319 cm−1, 459 K) was reported for an FeII–DyIII–FeII complex. Find more world records from all branches of chemistry on the Records and Challenges platform of The Chemical Record.