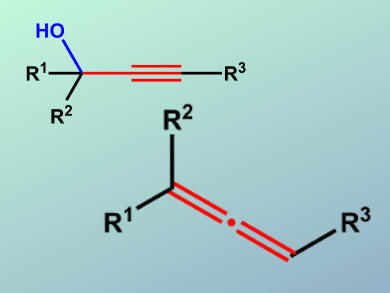
Allenes are important substrates and intermediates for organic synthesis and are found in many biologically active natural products. Therefore, the development of new methods for allene synthesis from easily available starting materials is important. Reductive deoxyallenylation of propargylic alcohols represents a convenient approach to allenes; however, the application of this method to sterically hindered tertiary propargylic alcohols is challenging.
Xihe Bi, Northeast Normal University, Changchun, China, and colleagues have developed a valuable methodology for the reductive deoxyallenylation of sterically hindered propargylic alcohols. This strategy relies on the combined use of Lewis and Brønsted acid catalysts in the reaction of hindered propargylic alcohols with 2-nitrobenzenesulfonylhydrazide (NBSH).
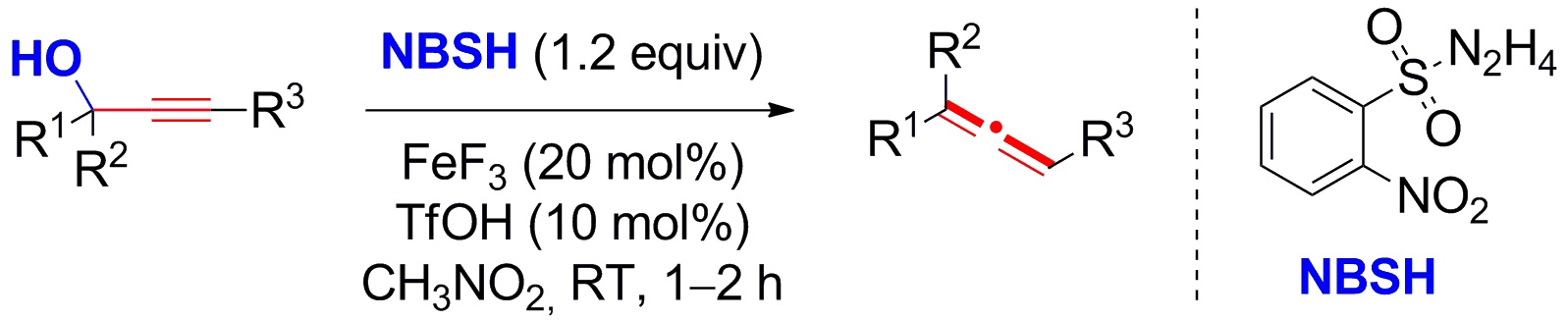
A wide variety of tertiary and secondary propargylic alcohols can be used, providing a range of mono-, di-, and trisubstituted allenes in good to excellent yields. The synthesis of 2H-chromenes and 1,2-dihydroquinolines further demonstrated the synthetic utility of this method. Based on the mild reaction conditions, broad substrate scope, and good functional-group tolerance, the researchers suggest that this methodology will find many applications in organic synthesis.
- Lewis and Brønsted Acid Cocatalyzed Reductive Deoxyallenylation of Propargylic Alcohols with 2-Nitrobenzenesulfonylhydrazide,
Zhaohong Liu, Peiqiu Liao, Xihe Bi,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404692