This week, D. P. Fairlie et al. review how to constrain cyclic peptides to mimic protein structure motifs. How does this lead to protease-resistant, potent and target-selective, biologically active compounds? In a Minireview, H. Mutlu and J.-F. Lutz discuss the sequencing of natural and synthetic macromolecules. The Highlights deal with the iron-catalyzed hydrogenation of esters to alcohols (J.-L. Renaud et al.) and protein crystallography with free-electron lasers (H. Ogata and W. Lubitz).
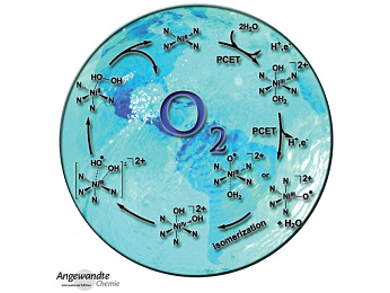
In the Communications section, T.-B. Lu et al. describe homogeneous electrocatalytic water oxidation at neutral pH by a nickel(II) complex (see picture). T. Taniguchi and D. P. Curran succeeded in the hydroboration of arynes with N-heterocyclic carbene boranes. B. P. T. Fokwa et al. found an unexpected synergy between magnetic iron chains and stacked B6 rings in a new ferromagnetic material. G. Gescheidt et al. investigated radicals from green-tea polyphenols and highly reactive oxygen species by time-resolved EPR spectroscopy.
- Angewandte Chemie 48/2014: Unexpected Synergy,
Angew. Chem. Int. Ed. 2014, 53 (48).