Photoactivatable fluorophores switch from a nonemissive state to an emissive form under illumination at an appropriate activation wavelength. By this, they emit light in the form of fluorescence upon irradiation at a suitable excitation wavelength. The ability to switch fluorescence on under optical control permits to reconstruct of fluorescence images with subdiffraction resolution as well as to monitor of dynamic processes in real time. As a result, photoactivatable fluorophores are valuable molecular probes for the visualization of the structural features and dynamic properties of diverse biological specimens and synthetic materials.
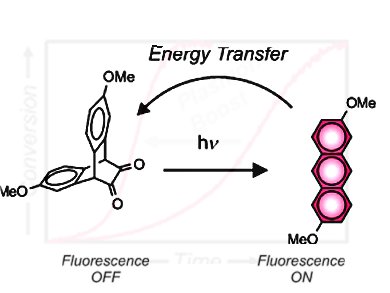
Françisco M. Raymo and co-workers at the University of Miami, FL, USA, developed photoactivatable fluorophores capable of sensitizing their own formation. A non-emissive reactant (R) is designed to absorb an activating photon and, after the decarbonylation of an α-diketone bridge, produce an anthracene fluorophore. The emissive product (P) absorbs another incoming photon and transfers its excitation energy to another molecule of the reactant to induce the formation of another copy of itself and establish an autocatalytic loop. Furthermore, plasmonic effects can be exploited to enhance the efficiency of energy transfer, if the photochemical reaction is performed in proximity to silver nanoparticles. This accelerates the autocatalytic transformation.
Thus, these innovative operating principles for photochemical replication can be particularly valuable to realize efficient photoactivatable fluorophores and to investigate the influence of plasmonic effects on the photochemical and photophysical properties of organic chromophores.
- Plasmonic Acceleration of a Photochemical Replicator,
Ek Raj Thapaliya, Burjor Captain, Françisco M. Raymo,
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402211