Proline is arguably the most important amino acid. However, the direct functionalization of proline is surprisingly little explored. Zhanxiang Liu and Yuhong Zhang, Zhejiang University, Hangzhou, China, and colleagues have developed a highly effective synthesis of C-3-substituted prolines. They used Pd-catalyzed C(sp3)–H activation for the straightforward functionalization of prolines.
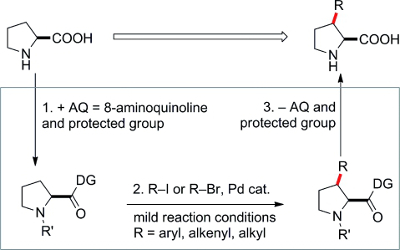
The use of an 8-aminoquinolinecarboxamide directing group allows direct arylation, alkenylation, and alkylation at the C-3 position of prolines in moderate to high yields with diverse iodo- or bromo precursors. This method should open up new avenues of research in biological chemistry and organocatalysis involving proline.
- Efficient Synthesis of cis-3-Substituted Prolines by Bidentate-Assisted Palladium Catalysis,
Ruokun Feng, Binjie Wang, Yue Liu, Zhanxiang Liu, Yuhong Zhang,
Eur. J. Org. Chem. 2014.
DOI: 10.1002/ejoc.201403191