Transformations of the azido group that are accompanied by the elimination of N2 (such as the Staudinger, Curtius, Schmidt, and Boyer reactions) have been used for the efficient formation of synthetically and biologically important compounds such as amines, imines, amides, and various azaheterocycles. Among the reactions of azides that take place without the loss of N2, the dominant reaction is the [3+2] cycloaddition of azides affording triazole and tetrazole derivatives.
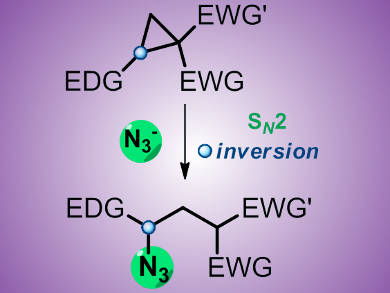
Ekaterina M. Budynina, Igor V. Trushkov, and colleagues, Moscow State University, Russia, have developed a new general method for azide synthesis that involves ring opening of donor–acceptor substituted cyclopropanes with the azide ion, providing straightforward access to libraries of polyfunctional azides of broad structural diversity in up to 91 % yield. Exceptional regioselectivity and stereospecificity of the ring opening is achieved by significant polarization of the C1–C2 bond in the three-membered ring and by the SN2-like mechanism of the reaction, which is supported by DFT calculations.
The high synthetic utility of these azides was demonstrated by transformations into five-, six-, and seven-membered N-heterocycles, as well as complex annulated compounds, including natural products and drugs, such as (–)-nicotine and atorvastatin.
- Ring Opening of Donor–Acceptor Cyclopropanes with the Azide Ion: A Tool for Construction of N-Heterocycles,
Konstantin L. Ivanov, Elena V. Villemson, Ekaterina M. Budynina, Olga A. Ivanova, Igor V. Trushkov, Mikhail Ya. Melnikov,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201405551



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)