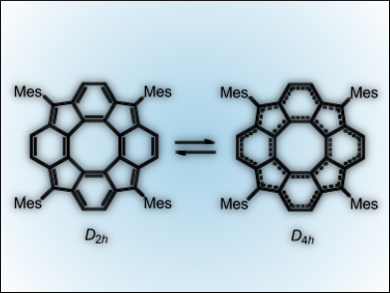
150 years after Kekulé rationalized the structure of benzene, aromatic and antiaromatic compounds are still of great interest. Yoshito Tobe and co-workers, Osaka University, Japan, have created a tetramesityl derivative of the previously unreported tetracyclopenta-[def,jkl,pqr,vwx]tetraphenylene (TCPTP). (Mes)4-TCPTP (pictured) was synthesized by the cyclization of a tetraketone with the central core and subsequent dehydrogenation.
This compound, which is a member of the corannulene family, is interesting for chemists as it can potentially adopt either open-shell tetra-radicaloid D4h, diradicaloid C2v, or closed-shell D2h structures. NMR studies showed that it exists as D2h structures that rapidly equilibrate via the D4h structure. The electronic structure involves 28 π-electrons, which are distributed in 8-π electron (inner) and 20-π electron (outer) systems. The latter gives the molecule its antiaromatic character.
Further studies are underway to force the molecule to adopt the D4h structure.
- Tetracyclopenta[def,jkl,pqr,vwx]tetraphenylene: A Potential Tetraradicaloid Hydrocarbon,
Shunpei Nobusue, Hirokazu Miyoshi, Akihiro Shimizu, Ichiro Hisaki, Kotaro Fukuda, Masayoshi Nakano, Yoshito Tobe,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201410791