Mankind consumes a great deal of tobacco, most commonly in the form of cigarettes. Worldwide, roughly 15,000 kg of nicotine from tobacco makes its way daily into smokers’ lungs. That alone is sufficient reason for us to consider this particular natural product more closely.
In this part we look at cigarette paper and additives, and at what happens when a smoker tries to quit.
15. All Cigarettes are Not Alike
Most cigarette smokers remain true to their chosen brand over the course of many years. This could perhaps be taken as evidence for subtle but distinct differences from one brand to another, but in any case, such brand loyalty demands of the manufacturer that every year, regardless of the nature of the tobacco harvest, each brand maintain its characteristic flavor as far as possible. Manufacturers expend considerable effort in their attempt to offer products that show such consistency of taste.
15.1. Tobacco Mixtures
To begin with, each kind of cigarette entails its own unique mixture of tobaccos, purchased as batches of tied bundles, each weighing several kilograms. Different lots from a given harvest will, of course, differ somewhat, so the cigarette manufacturer must carefully analyze each lot, both chemically and on the subtle basis of flavor. The most important chemical parameters include nicotine content, of course, but also ammonium and nitrate levels, as well as those of various sugars. Lots are also classified with respect to taste, such that, for example, a producer might mix together batches with high and low nicotine contents, but also some from various taste categories, all in the effort to achieve a relatively consistent product with respect to nicotine level, but also flavor.
At the end of the production process, cigarettes themselves will be submitted to strict quality controls. For example, smoke from the resulting cigarettes, generated under internationally standardized conditions, is subject to laboratory analysis, starting with absorption on special filters. Gases like carbon monoxide simply pass through such filters, and must therefore be monitored separately. Residues collected in the filters are then extracted for detailed analysis. Finally, results for certain characteristic parameters are typically disclosed on the packaging [39].
15.2. Cigarette Filters and Paper
Additional control factors are the filter and papers utilized, since these significantly influence air delivery and the rate of combustion. Thus, a cellulose acetate filter might be altered so as to make it slightly longer, shorter, or firmer, or even discontinuous, and there are virtually no limits with respect to the nature of the cigarette paper, apart from imagination.
In terms of flavor and its intensity, paper porosity plays a critical role, since this greatly influences the amount of air that will be sucked in. Air permeability of the cigarette paper also has a substantial effect on the amount of condensate and nicotine the smoker takes in, in turn affecting the perceived flavor. Special papers are required in cigarette production. To begin with, distinctions must be made between the paper for the body of the cigarette, that for the tip (“mouthpiece”), and that intended to encase the filter. Requirements for each are distinct.
Given a production rate of up to 16,000 cigarettes per minute, all paper employed is subject to severe demands with respect to mechanical stability and stretchability. The paper is drawn at extremely high rates of speed from rolls that may hold up to several kilometers of stock. The paper is rather porous, so it directly influences how much air will be available for combustion. Weight of the basic paper is generally only about 24–37 g/m2, and it displays a porosity of 30 – 110 CU, where “CU” refers to “CORESTA unit”, a standard unit of measure for porosity. CORESTA (Centre de Coopération pour les Recherches Scientifiques Relatives au Tabac) is a combination of representatives from the tobacco industry and from institutions that deal with tobacco and tobacco production. A CU value indicates how many cm3 of air per minute will pass through paper with a surface area of 1 cm2 under a pressure equivalent to a 10 cm water column.
Many countries have implemented policies only allowing the sale of cigarettes with “reduced ignition potential”, primarily in the interest of preventing fires – due, for example, to someone forgetting to extinguish a burning cigarette. These “fire-prevention cigarettes” have two sprayed-on strips of either alginate or cellulose on the inner side of their paper.
Paper for the cigarette tip is typically imprinted with brown ink, often so patterned as to mimic the look of cork. This paper plays an important role in regulating the amount of excess air introduced, often through tiny perforations that may or may not be readily visible to the naked eye. In the case of cigarettes supposedly producing low levels of nicotine and tar (condensate), closing off these perforations with the fingers can result in delivery of considerably more of both of these substances.
The type of paper surrounding the filter has a major effect on perceived “strength” of a cigarette. This paper may have a high permeability to air, since it is the air supply at this point that is the principal determinate of “strength” in this sense. The actual porosity of the paper here can in fact be exceedingly high: as great as 20,000 CU.
15.3. Additives
Apart from the tobacco blend, the paper, and the filter, various additives can also influence the flavor and descriptive parameters for a cigarette. The permissibility of additives may be strictly regulated, however, and – at least in Germany – it may be required that their presence be disclosed [40].
Additives such as sugar, chocolate, or licorice are used to cause smoke to seem milder and more pleasant. Another common additive is menthol, sometimes added in small amounts even to cigarettes not labeled als “menthols”. Menthol produces a mild cooling effect in the smoker’s airway, expands the small endings in the bronchial tubes, and also acts as a mild anesthetic. An important consequence is that the smoker is able to inhale more deeply, thereby hastening and intensifying nicotine uptake. Prohibition of menthol as an additive has long been under discussion in the EU, but a formal decision has regularly been deferred due to pressure exerted by various special interest groups.
15.4. Nicotine Uptake Depending on pH
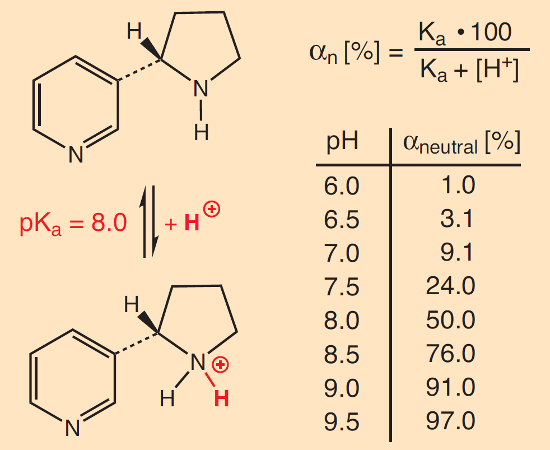
The discussion has been especially controversial regarding additives that can thermally generate ammonia. By increasing the pH of the smoke, the percentage of the neutral form of nicotine in the smoke is raised (Fig. 13) [41]. This can have important consequences, since nicotine is unable to pass through the cell membranes of pulmonary alveoli and blood vessels in the protonated form. Any increase in pH thus facilitates and accelerates the uptake of nicotine, which in turn increases the risk of addiction [42].
|
|
|
Figure 13. Nicotine as an acid–base system. |
Tobacco smoke is, of course, not an aqueous solution, but an aerosol, and thus a complex multiphase system of inhomogeneous composition. Determining the pH of an aerosol is no trivial matter. A truly original approach to measuring the fraction of nicotine present in its neutral form in such an aerosol has been developed by James F. Pankow and his associates [43]:
- With the aid of a “cigarette-smoking machine”, smoke equivalent to several cigarette puffs is collected.
- A first sample of the combined smoke is drawn through a Teflon membrane, which is permeable only to the neutral form of nicotine, but impermeable to the protonated form.
- After passage through the membrane, all organic components of the gas are precipitated virtually quantitatively onto an adsorbent.
- These adsorbed substances are then desorbed by warming, and their nicotine content is determined. This leads to a concentration c0 for the neutral form of nicotine in the tobacco smoke.
- The entire analysis is then repeated with a second sample of the collected smoke, but this time the smoke sample is treated with an excess of gaseous ammonia beforehand. This ensures that all the nicotine present will be converted to its neutral form. The “basified” smoke sample is then subjected analogously to determine the total nicotine content, ctotal.
- From the ratio of the two results, c0/ctotal, one can derive a value for the fraction of smoke-based nicotine originally in its neutral form, αN = 100 c0/ctotal, from which it becomes easy, in turn, to compute a pH value for the smoke (see Fig. 13).
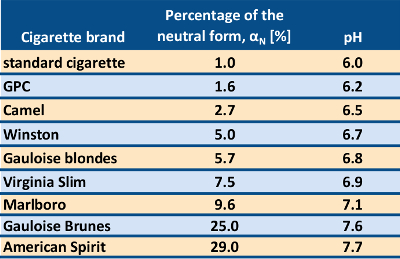
A set of such data obtained from a variety of cigarette brands is rather striking! The examples shown in Table 2 start with an effective pH value of 6.0 obtained in this way from what the University of Kentucky, Lexington, USA, regards as a “reference cigarette”. This has also been taken elsewhere in the literature to be “typical” for cigarette smoke. It will be seen, however, that most American cigarette brands actually result in significantly higher values. For example, a pH for the Marlboro brand was found to be above 7, representing a relative decrease in proton concentration by a factor of 10, or a tenfold increase in the concentration of the neutral form of nicotine.
|
Table 2. Effective pH values for tobacco smoke from various cigarette brands. |
|
|
It is obvious that such an indirect pH determination should – in fact, must – be subjected to critical scrutiny by the scientific community, and opened to confirmation or doubt on the basis of further study: a perfectly normal process within the experimental sciences. At the same time, conflicts of interest are difficult to rule out completely with respect to many research publications, especially if (in this particular instance, for example) the relevant work was financed directly by the cigarette industry [44,45].
16. The Prolonged Goodbye to Cigarettes
Roughly 70 % of all smokers admit they would like to give up the habit completely, and about 46 % have actually tried to do so at least once in the last 12 months, albeit in vain [46]. Cigarettes continue to be tempting. Most smokers start to experience withdrawal symptoms within as little as a few hours after the onset of nicotine deprivation. Complaints include anxiety, nervousness, difficulty concentrating, and irritability.
16.1. Resources for Nicotine Withdrawal
Since for most smokers quitting tends to be so difficult, pharmaceuticals that supposedly help with nicotine withdrawal have been on the market for many years. The usual strategy is to provide a temporary and gradually diminishing substitute for the nicotine source responsible for dependency in the first place, thereby alleviating the withdrawal symptoms somewhat. Industry has developed a wide array of such nicotine-containing substitutes, including chewing gums, patches, and lozenges designed to support smokers during the withdrawal period (Table 3). Nicotine itself, at the dosage smokers typically encounter it (maximum 1 mg per cigarette), has a minimal detrimental effect on one’s health, so continuing to supply a little nicotine as part of a withdrawal strategy – in the absence of the other harmful substances present in tobacco smoke – seems certainly acceptable.
|
Table 3. Resources for nicotine withdrawal — or supports for nicotine addiction? |
||||||||||
|
16.2. Weight Gain During Nicotine Withdrawal
Even with these resources, the smoker wishing to quit will still need to withstand temptations, taking a variety of forms. Especially dangerous are excuses with a pseudoscientific origin. The most popular one involves the weight gain phenomenon widely experienced at the start of withdrawal. Fear of “getting fat” is frequently used as a pretext for once more taking up the habit. This irrational behavior is based on the one hand on today’s peculiar ideal of “beauty” (in turn derived from severely undernourished models, some of whom might in fact need clinical treatment), and on the other hand on the fallacious notion that weight gain carries a greater risk to one”s health than smoking does [48]. It only recently became possible to provide a biochemical explanation for nicotine’s long-recognized appetite suppression [49]. This is a phenomenon worthy of a somewhat closer examination.
The longing for food is associated with a very complex regulation process, involving many physiological parameters and signaling molecules [50, 51]. The true control center is located in the Nucleus arcuatus portion of the hypothalamus, a tiny region in the diencaphalon or interbrain. The crucial molecular sensor is the MC4 receptor, localized on neurons in this area. This sensor is activated by the peptide hormone α-MSH, consisting of 13 amino acids. α-MSH is released by the neighboring POMC (pro-opiomelanocortin) neurons when these are themselves stimulated. These POMC neurons produce pro-opiomelanocortin, a precursor polypeptide consisting of 241 amino acids. The molecule is a biochemical “multitalent”, in that, depending upon the species and type of tissue, it can be cleaved into any of 11 different peptides, fulfilling totally different functions. α-MSH is only one of these peptides.
Here, nicotine finally gets involved: It binds to α3β4-nicotinic acetylcholine receptors of POMC neurons, thereby activating them. This causes α-MSH to be released, which then binds to the MC4 receptors (Fig. 14). The resulting neuronal signal is passed along to the central nervous system, and interpreted there as an indication of satiation. One could say, in simplified form, that nicotine causes one neuron to send a molecular message to another nearby neuron, which in turn sends another message to the central nervous system, which the latter understands to be a sign the person is no longer hungry.
|
|
|
Figure 14. Nicotine as an appetite suppressant. |
In other words, increased consumption of food in the early phases of nicotine withdrawal is not a psychopathological flight from one addiction to another, but rather the result of sudden loss of the accustomed appetite suppressor – nicotine – upon which the body (as one of the consequences of chronic smoking) has learned to rely. Only after passage of a little time will the internal control circuit readjust itself to its original, nicotine-free set-point.
17. The Dilemma Created by Cigarettes
It took several centuries for the health hazards related to smoking to be clearly recognized. This has now brought before the public the dilemma of somehow balancing an immense loss of income, long provided by tobacco taxes, against grave (and costly!) health risks confronting the same country’s own citizens. Rudimentary solutions developed in the past have never gotten mankind very far. For example, Sultan Murad IV of the Ottoman Empire (1612 – 1640) attempted to deal with smoking through brute force: over the course of five years he simply had 25,000 smokers executed. But smoking persisted anyway. As early as 1702 in the German province of Baden, the alternative of digging deeply into the smokers’ pockets was pursued, via decree: “Everyone who thus drinks [sic] tobacco will be subjected to a fiscal penalty so that, little by little, armamentaria for martial purposes can be purchased” [2]. But that didn’t work either.
Perhaps scientific insights accumulated in recent decades can offer a certain amount of hope here. Native American tribes incorporated smoke into their ritualistic activities, and smoking retains a certain amount of ritual even today. In the 17th century, people puffed away at tobacco in pipes; in the 18th century, snuff and snuffing was all the rage; in the 19th century, it took the form of cigars; and in the 20th century, cigarettes (see Part 1 of this series). How will smoking evolve for the 21st century?
One clue may come from the 2012 Business Review of British American Tobacco, one of the largest cigarette producers worldwide [52]: “The scientific community widely agrees that it is the toxicants in tobacco and tobacco smoke, not the nicotine, that causes the majority of tobacco-related diseases. Conventional cigarettes carry the most risks to health, while some forms of low-toxicant smokeless tobacco products, such as Swedish-style snus, although not risk-free, are much less risky. Regulatory-approved nicotine products that contain no tobacco or smoke toxicants are almost risk-free.”
“The UK’s Royal College of Physicians has said ‘if nicotine could be provided in a form that is acceptable and effective as a cigarette substitute, millions of lives could be saved.’ We encourage tobacco companies, scientists and regulators to work together to ensure a science-based approach is used to assess new products that potentially pose less risk. This will provide consumers with the assurance that the product information they receive is based on sound science and allows them to make an informed choice based on the risk profile of different products [52].” E-cigarettes are currently marketed as a healthier alternative to tobacco in the UK.
References
[39] Reemtsma GmbH, How are condensates and carbon monoxide measured? (in German).
[40] German Federal Ministry of Food and Agriculture, List of all tobacco products traded in Germany and their additives (in German).
[41] J. H. Summerfield, J. Chem. Educ. 1999, 76, 1397. DOI: 10.1021/ed076p1397
[42] German Cancer Research Center, Health risks of tobacco additives: Ammonium compounds (in German).
[43] J. F. Pankow et al., Chem. Res. Toxicol. 2003, 16, 1014. DOI: 10.1021/tx0340596
[44] J. F. Seeman, J. Chem. Educ. 2005, 82, 1577. DOI: 10.1021/ed082p1577
[45] J. F. Seeman, Chem. Res. Toxicol. 2007, 20, 326. DOI: 10.1021/tx600290v
[46] Centers for Disease Control and Prevention, Cigarette Smoking Among Adults, MMWR Weekly, 1997.
[47] German Federal Institute for Risk Assessment, Liquids from e-cigarettes can have health risks (in German).
[48] D. F. Williamson et al., New Engl. J. Med. 1991, 324, 739. DOI: 10.1056/nejm199103143241106
[49] Y. S. Mineur et al., Science 2011, 332, 1330. DOI: 10.1126/science.1201889
[50] M. W. Schwartz et al., Nature 2000, 404, 661. DOI: 10.1038/35007534
[51] K. L. J. Ellacott et al., Recent Prog. Horm. Res. 2004, 59, 395. ISBN: 0-879225-51-4
[52] British American Tobacco, Annual Report 2012.
The article has been published in German as:
- Starker Tobak – Unsere Lust und Last mit der Zigarette,
Sabine Streller, Klaus Roth,
Chem. Unserer Zeit 2013, 47, 248–268.
DOI: 10.1002/ciuz.201300636
and was translated by W. E. Russey.
The Chemistry of Tobacco – Part 1
Looking at the history of tobacco consumption – from chewing and snuffing to smoking
The Chemistry of Tobacco – Part 2
What does tobacco contain and which chemical changes happen between the harvest and a finished cigarette?
The Chemistry of Tobacco – Part 3
How does a tobacco plant synthesize nicotine?
The Chemistry of Tobacco – Part 4
What does cigarette smoke contain and what does nicotine do to the smoker?
See all articles by Klaus Roth published by ChemViews magazine
.jpg)


