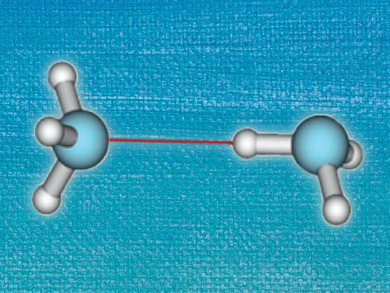
Hydrogen bonds are important aspects of many chemical and biological systems. Since systems of interest often contain a large number of hydrogen bonds, small imprecisions for single interactions can add up to large errors. Accurately describing these weak interactions computationally usually requires time-intensive methods.
In contrast, Density Functional Theory (DFT) allows the treatment of large systems at acceptable computational costs, but does not necessarily describe weak interactions with the required accuracy. So-called dispersion-corrected DFT (DFT-D) deals with this issue by including correction terms for van der Waals interactions. However, do dispersion corrections improve calculations on hydrogen-bonded systems?
A. Daniel Boese, University of Graz, Austria, reports on the performance of DFT with and without dispersion corrections. Using a large set of different functionals, he has performed calculations on hydrogen-bonded systems in a range of different sizes, from the HF dimer to peptide complexes, and compared the results to reference values generated by highly accurate Coupled Cluster calculations. It turns out that including dispersion corrections for small hydrogen-bonded systems of four to eight atoms actually increases the mean error. This is opposed to larger organic molecules, where van der Waals interactions play a more important role and DFT-D methods lead to improved performance.
- Density Functional Theory and Hydrogen Bonds: Are We There Yet?
A. Daniel Boese,
ChemPhysChem 2015.
DOI: 10.1002/cphc.201402786