Indole and pyrrole units are common motifs in bioactive molecules and new approaches to their selective C–H functionalization are in constant demand. The atom-economical route of dehydrogenative coupling of two (hetero)arene C–H units often presents regioselectivity or scope limitations, the need for a large excess of one coupling partner, harsh conditions, or requires expensive additives. Thus, organoboronates are an attractive alternative for oxidative direct arylation reactions. However, Ru-catalyzed C–H functionalization reactions with boronates remain rare.
Lukasz Pilarski and colleagues, Uppsala University, Sweden, have explored the Ru-catalyzed C2–H arylation of indoles and pyrroles by using boronic acids under oxidative conditions. This reaction can be applied to tryptophan derivatives and tolerates a wide range of functional groups on both coupling partners, including those bearing nitro, ketone, alkenyl, silyl, sulfonyl, ferrocenyl, and both bromo and iodo groups.
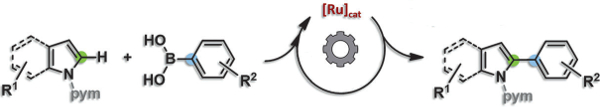
Tolerance of aryl iodides is both rare and complementary to their frequent use as oxidants or electrophiles in C–H arylation reactions, allowing further selective derivatization. Mechanistic studies suggest the catalyst loses its cymene ligand and that the on-cycle metalation occurs through an electrophilic attack by the Ru center. Efforts to broaden this methodology to other transformations and substrates are ongoing.
- Ru-Catalysed C–H Arylation of Indoles and Pyrroles with Boronic Acids: Scope and Mechanistic Studies,
Carina Sollert, Karthik Devaraj, Andreas Orthaber, Paul J. Gates, Lukasz T. Pilarski,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201405931