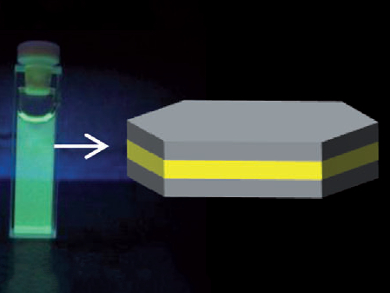
Encapsulation of Functional Organic Molecules
Many applications demand the presence of functional organic molecules in environments where they are not stable or even not soluble. A possible way to protect the molecules is encapsulation in materials that provide solubility but do not impair the functionality. In this context, the teams led by Josef Breu, Stephan Förster, Hartmut Yersin, and Geoffrey A. Ozin at the Universities of Bayreuth, Regensburg, both Germany, and Toronto, Canada, have produced nanometer-sized double stacks of a transparent silicate material having a central layer of oriented hydrophobic fluorescent dye molecules. The nanoplatelets are well dispersed in aqueous solution, and can be cast into dry films that feature almost perfect texture and are optically anisotropic.
Immobilizing Dye Between Silicate Layers
The scientists emphasize that their novel dye-encapsulating double stacks were based on their former detailed studies on sodium hectorite, a layered silicate material that can be melt-synthesized with perfectly homogeneous charge density. Organic compounds can be intercalated in the layers, and the fluorescent stilbazolium dye N-hexadecyl-4-(3,4,5-trimethoxystyryl)-pyridinium forms a perfectly ordered heterostructure the layers of which are arranged in a strictly alternating order of hydrated sodium ions, silicate, and dye. However, it came as a surprise that “immersing the microcrystalline powder of the ordered interstratified heterostructure into deionized water results in the material swelling …,” the scientists write. This huge increase in sample volume indicated “a delamination of the one-dimensional crystals into thinner platelets.”
When they cast diluted suspensions of these platelets on a flat substrate, a film was developed that showed the same wettability as the pristine silicate, which meant that the hydrophobic molecules were completely immobilized between the silicate layers. Investigating the platelets by atomic force microscopy, the researchers found a typical diameter of 5 μm at a height of 4.5 nm corresponding to a stacking order of silicate (0.96 nm) – hydrated sodium ions (0.54 nm) – dye (1.6 nm) – hydrated sodium ions – silicate, a finding that was further supported by small-angle X-ray scattering studies. The monomolecular dispersion of the dye between the “hamburger” double stacks was shown by the homogeneous strong fluorescence of the diluted transparent suspension.
Possible Applications in Functional Surfaces
The hybrid silicate functional organic molecule double stacks can be utilized in general applications, because they remain stable and functional if compounded into a polymer matrix, the authors wish to highlight. As a proof of principle, they produced a nanocomposite film that displayed the perfect texturing of the original silicate sheet. The confined dye molecules still had their fixed orientation imposed by the structural anisotropy of the silicate layers as it was shown by UV/Vis polarized absorption spectra. Thus the new encapsulation strategy is suitable not only for medical applications, but also for surface coatings to achieve functional surfaces with anisotropic optical properties.
- Encapsulation of Functional Organic Compounds in Nanoglass for Optically Anisotropic Coatings,
M. Stöter, B. Biersack, S. Rosenfeldt, M. J. Leitl, H. Kalo, R. Schobert, H. Yersin, G. A. Ozin, S. Förster, J. Breu,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201411137