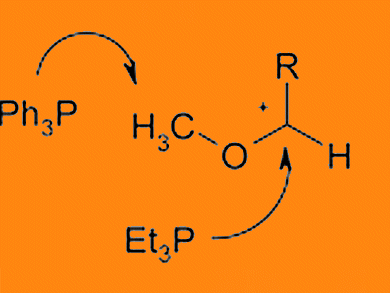
Triethylphosphane hydrobromide in its reaction with dimethyl acetals leads to α-methoxy phosphonium salts as has been reported by Priyabrata Das and James McNulty, McMaster University, Ontario, Canada. However, under the same conditions, triphenylphosphane hydrobromide was shown to be methylated giving the corresponding quaternary methyl(triphenyl) phosphonium salt.
They also report that the ylide formation/olefination reactions from the α-methoxy phosphonium salts formed in this manner, could be employed in the synthesis of a wide range of vinyl ethers and variously functionalized 1,3-dienes.
The production of quaternary phosphonium salts using this general P-methylation process avoids the use of toxic alkylating agents. Further investigations into the preparation and reactivity of these salts and applications of the vinyl ether products are under investigation.
- Dichotomous Reactivity in the Reaction of Triethyl- and Triphenylphosphane HBr Salts with Dimethyl Acetals: A Novel Entry to α-Alkoxy-Functionalized Ylides and General Synthesis of Vinyl Ethers and Alkoxy Dienes
P. Das, J. McNulty,
Eur. J. Org. Chem. 2010, 2010(19), 3587 – 3591.
DOI: 10.1002/ejoc.201000601