Schilancitrilactones B and C were isolated in 2012 by Sun and co-workers from the stems of Schisandra Lancifolia [1], which have been used in traditional Chinese medicine for the treatment of neurasthenia and related diseases. Preliminary biological assays indicated that schilancitrilactone C showed biological activities for inhibiting HIV-1 while schilancitrilactone B was not bioactive.
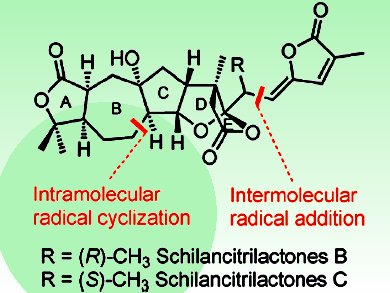
Pingping Tang and colleagues, Nankai University, Tianjin, China, have accomplished the first total syntheses of schilancitrilactones B and C. They started from commercially available materials and needed 17 steps (longest linear sequence). The key steps include the successful implementation of an intramolecular radical cyclization to prepare a seven-member ring, late-stage iodination, and an intermolecular radical C–C bond formation.
The researchers say that their approach provides a sequence for the syntheses of compounds related to the schilancitrilactones, as well as their derivatives and analogues.
- Total Synthesis of Schilancitrilactones B and C,
Liang Wang, Hengtao Wang, Yihang Li, Pingping Tang,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201501169
[1] Schilancitrilactones A–C: Three Unique Nortriterpenoids from Schisandra lancifolia,
Xiao Luo, Yi-Ming Shi, Rong-Hua Luo, Shi-Hong Luo, Xiao-Nian Li, Rui-Rui Wang, Sheng-Hong Li, Yong-Tang Zheng, Xue Du, Wei-Lie Xiao, Jian-Xin Pu, Han-Dong Sun,
Org. Lett. 2012, 14, 1286–1289.
DOI: 10.1021/ol300099e