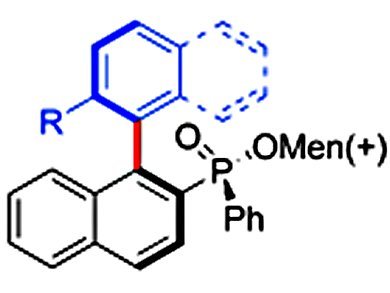
Transformations resulting in the enantioselective formation of C—C bonds are widely sought after for the synthesis of biologically active natural products. Chiral ligands play a crucial role in the process of controlling enantioselectivity in these reactions. Of all of the ligands used in this way, chiral phosphines have proven to be among the most effective and selective for a wide variety of enantioselective transition-metal-catalyzed reactions, with many bidentate phosphine ligands designed and applied to asymmetric synthesis. However, only a limited number of monodentate chiral phosphine ligands have been reported for C—C bond-forming reactions, despite certain metal-catalyzed reactions proceeding smoothly and with high enantioselectivity if monophosphine ligands are used.
Yan-Na Ma and Shang-Dong Yang, Lanzhou University, China, have addressed this imbalance by utilizing a menthyl phenylphosphinate-assisted asymmetric Suzuki-Miyaura cross-coupling reaction to successfully synthesize a series of chiral biaryl compounds with a phosphinate group as a chiral auxiliary in good yields and with excellent diastereomeric ratios (up to >95:5 d.r.).
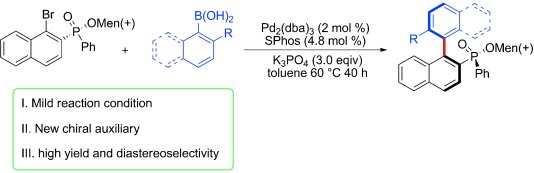
This methodology provides a highly efficient and practical strategy for the synthesis of new axially chiral biaryl monophosphine oxides and their corresponding phosphines. The application of these monophosphine ligands in asymmetric transition-metal-catalyzed reactions is currently under investigation by the group.
- Asymmetric Suzuki—Miyaura Cross-Coupling for the Synthesis of Chiral Biaryl Compounds as Potential Monophosphine Ligands,
Yan-Na Ma, Shang-Dong Yang,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201406554