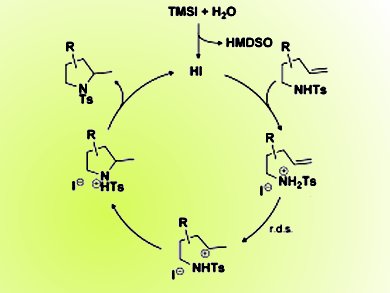
Lycopodium alkaloids possess interesting biological activity as well as challenging structural complexity, which make them attractive synthetic targets. Over the last ten years the group of Richmond Sarpong at the University of California, Berkeley, USA, have been interested in the total synthesis of members of this class of natural products. They have investigated the use of an advanced “Heathcock-type” 6–5–9 tricycle intermediate in their efforts to access a range of Lycopodium alkaloids.
.jpg)
Whilst studying this intermediate they observed an unexpected transannular hydroamination (pictured above) reaction mediated by “anhydrous” hydrogen iodide (HI) to provide the tetracyclic core of serratine and 8-deoxyserratine. They then decided to investigate the limitations and scope of this fortuitous reaction and have demonstrated the importance of the iodide anion in promoting this transformation and have been able to expand the reaction into a general method:

Owing to the synthetic ease of generating “anhydrous” HI (generated in situ from trimethylsilyl iodide and water) as well as the effectiveness of this hydroamination method, they believe it will find broad utility in the future development of acid-catalyzed carbon–heteroatom bond-forming reactions.
- Synthetic Efforts toward the Lycopodium Alkaloids Inspires a Hydrogen Iodide Mediated Method for the Hydroamination and Hydroetherification of Olefins,
Paul R. Leger, Rebecca A. Murphy, Eugenia Pushkarskaya, Richmond Sarpong,
Chem. Europ. J. 2015, 21, 4377–4383.
DOI: 10.1002/chem.201406242
If you are interested in finding out more, Richmond Sarpong will be giving a talk on “Strategies and tactics for chemical synthesis inspired by complex alkaloids” at the 249th ACS National Meeting & Exposition in Denver, USA, in the Four Season Ballroom 2&3, Colorado Convention Center on Tuesday, March 24 at 2:20pm.