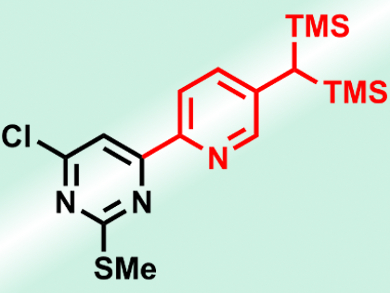
Heterocyclic rings are found in many naturally occurring compounds and methods for their regioselective preparation are of great interest to the chemical community. Regioselective functionalization of heterocycles by metalation reactions is one such method. Recently, the bis(trimethylsilyl)methyl (BTSM) protecting group has been used for the efficient regioselective metalation of aromatic substrates, as well as for that of a few heterocycles, such as pyrazines.
Paul Knochel and co-workers, Ludwig-Maximilians-Universität München, Germany, extend this methodology by a simple procedure for the preparation of functionalized BTSM-substituted heteroaryls. They use either a Kumada–Corriu or Negishi cross-coupling reaction.
The reaction tolerates a broad scope of functional groups, such as esters, ketones, or even aldehydes, and the resulting BTSM-substituted N-, O-, or S-heterocycles were metalated regioselectively with different Li or Mg bases in a site-selective manner. After quenching with a variety of different electrophiles, highly substituted heterocycles were produced in good yields (example pictured). The group then subjected these heterocycles to transformations leading to the corresponding methyl-, formyl-, or styryl-substituted heteroaryls.
- Preparation and Regioselective Magnesiation or Lithiation of Bis(trimethylsilyl)methyl-Substituted Heteroaryls for the Generation of Highly Functionalized Heterocycles,
Thomas Klatt, Veronika Werner, Marina G. Maximova, Dorian Didier, Yitzhak Apeloig, Paul Knochel,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500627