1. The Second Semiconductor Revolution
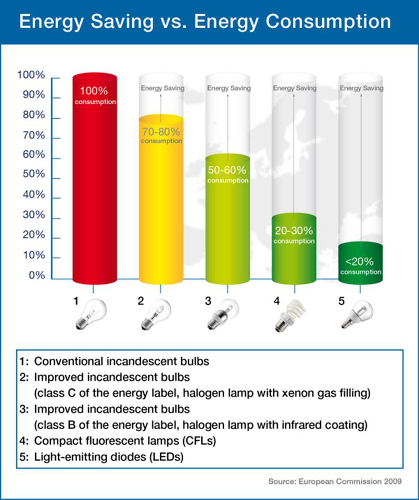
Today, artificial lighting accounts for up to 20 % of the energy consumed in private households [1]. Whereas the luminous efficacy of incandescent light sources is approximately 15 lm W–1, compact fluorescent lamps (CFL) reach up to 70 lm W–1. These lamps are fabricated at the technological limit; therefore, only small performance improvements might be expected. This is different for light sources based on light-emitting diodes (LED). LEDs represent a highly energy efficient lighting technology and have the potential to fundamentally change the future of artificial lighting (Fig. 1).
|
|
|
Figure 1. Potential energy savings by use of LEDs. |
The theoretical limit for a LED-driven white light source with a color rendering index (CRI) of 90 and a correlated color temperature (CCT) of 3000 K is 408 lm W–1 [2]. Today, commercially available LED devices can reach up to 200 lm W–1, and their luminous efficacy is steadily increasing. This is reflected by the so-called Haitz Law [3]. It states that every decade the cost per lumen falls by a factor of ten, whereas the amount of light generated per LED package increases by a factor of 20.
The semiconductor of choice for these devices is AlGaInN. This material class displays a range of bandgaps, which allow the emission of photons over the entire visible range. However, the wall-plug efficiency is best in the blue spectral region with a maximum at approximately 420 nm. The efficiencies are dramatically worse for longer wavelengths and reach a minimum in the green to yellow parts of the spectrum (“yellow gap”) [4,5]. Therefore, a suitable luminescent material (“phosphor”) or a suitable blend of phosphors are combined with a blue-emitting diode (pcLEDs) to achieve a higher color rendering and a good adaptation to the photooptic sensitivity of the eye (Fig. 2).
|
|
|
Figure 2. Rendered volume of a state-of-the-art white pcLED obtained by X-ray computed tomography (CT) showing the yellow-emitting phosphor (light yellow) mounted to the blue-emitting LED chip (light blue). |
2. Generation of White Light: Luminescent Materials for pcLEDs
2.1 YAG:Ce – The Ideal LED Phosphor?
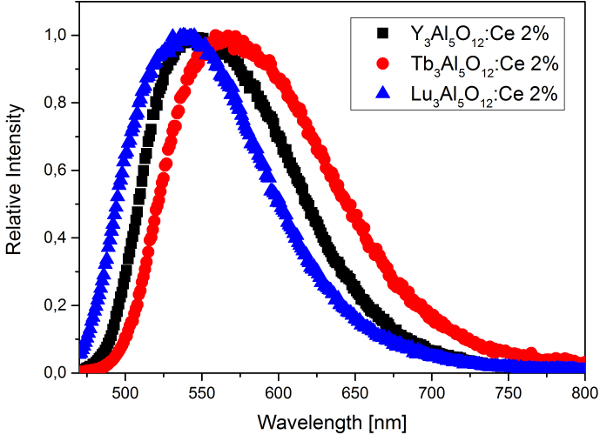
Today, almost all of the single-phosphor pcLEDs are based on Ce3+-doped yttrium aluminium garnet (Y3Al5O12; YAG:Ce) [6,7]. This material fulfills all the requirements for LED phosphors and, therefore, can be considered an archetypal LED-phosphor material [8,9]. The excitation spectrum for YAG:Ce, which peaks at 440–460 nm, is well matched to the emission of blue LEDs, and its yellow broadband emission in the visible spectral region (500–700 nm) provides a reasonable CRI. In addition to its high quantum yield and thermal quenching temperature, the emission color of YAG:Ce can be tuned by substitution of lattice constituents; for example, yttrium can be replaced by the smaller lutetium (the emission shifts to shorter wavelengths) or larger terbium (the emission shifts to longer wavelengths) (Fig. 3).
|
|
|
Figure 3. Emission spectrum of YAG:Ce and spectral shift of the emission owing to substitution of yttrium. |
Such a single-phosphor approach might be very simple and efficient, but it also has one major drawback: the limited spectral power distribution causes a CCT > 4000 K (cool white light) and a low CRI (usually below 80). For many applications—especially indoor illumination—a higher CRI and lower (i.e., warmer) CCTs in the range of 2700–3200 K are preferable. Suitable white-light LEDs can be produced by applying a second red-emitting phosphor in addition to YAG:Ce.
2.2 Eu2+-Activated Luminescent Materials
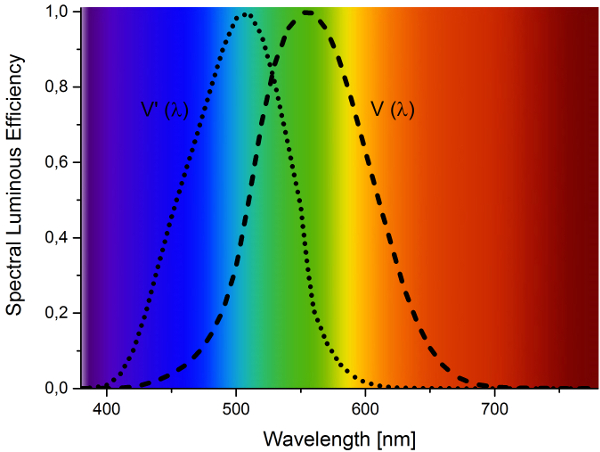
The efficient generation of white light by two phosphor pcLEDs always suffers from a tradeoff between high color rendition, low correlated color temperature, and luminous efficacy. To further improve the luminous efficacy, the emission of the red phosphor has to be optimally adapted to the sensitivity of the human eye (Fig. 4). To reach this goal it is mandatory to control the spectral peak position and width.
|
|
|
Figure 4. The photopic luminosity function (V(λ)) and scotopic luminosity function (V’(λ)) describing the average spectral sensitivity of the human eye. |
Nowadays the number of red-emitting luminescent materials suitable for commercial devices is rather small owing to the difficulty in meeting these requirements. The materials of choice are Eu2+-activated nitridosilicates such as (Ba, Sr)2Si5N8:Eu and (Ca,Sr)AlSiN3:Eu [10–13]. These materials offer a tunable emission color, good thermal behavior, high CRIs for a huge range of CCTs, broad excitation bands, and a high efficacy. However, the rather broad emission bands of the Eu2+-activated red-emitting materials always suffer from one problem: a substantial fraction of the light is emitted at wavelengths longer than 700 nm and is therefore beyond the sensitivity of the human eye. More recently, Sr[LiAl3N4]:Eu was presented as a narrow-band red-emitting material [14]. Replacing commercial red phosphors with Sr[LiAl3N4]:Eu showed a significant increase in the luminous efficacy of white pcLEDs.
2.3 Search for the “Holy Grail” – Line-Emitting Red Phosphors
Line-emitting luminescent materials in the red spectral region that can be activated by blue-emitting diodes are highly sought after owing to the possibility of the concentration of radiated energy in those parts of the visible spectrum that coincide with high eye sensitivity. They promise the greatest benefit to efficiency at high CRIs and low CCTs. Line emission in inorganic materials occurs if the activator emits at an energy level that is positioned vertically above the ground state in the configurational coordinate diagram. This is especially true for all f–f transitions, as the electronic levels are shielded from the chemical surroundings by the further outward-lying d states. However, the limitation of commercially available line-emitting red phosphors, e.g., Eu-activated Y2O3:Eu [15, 16], is the missing strong absorption bands in the blue spectral region.
In certain cases, transitions between different d states also exhibit line emission. This is a result of only minimal changes in the bonding strength and distance during the involved transition and of the fact that the electron configuration on the metal center remains unchanged.
An example of a transition metal that shows line emission in the deep red (>620 nm) on the basis of d transitions is the 3d3 ion manganese(IV). In contrast to f ions like the lanthanides, d ions might show relatively strong absorptions on the metal center, which are usually broad. Thus, efficient red line-emitting phosphors based on manganese(IV) activation might be possible.
2.4 Discovery of New Phosphors: High-Throughput Combinatorial Chemistry
Whereas classical research into new luminescent materials is time-consuming and cumbersome, a very promising new approach for the discovery of new phosphors was recently presented by Hirosaki et al. [17]. The use of single-particle diagnosis is very simple and saves a significant amount of time. For this, a luminescent particle that has been distinguished from a complex arbitrarily prepared powder mixture was characterized. Compared to classical solid-state reactions, it is not necessary to synthesize phase-pure powders or large single crystals, because photoluminescence properties can be characterized by single-particle fluorescence spectroscopy, and super-resolution single-crystal X-ray diffractometers enable analysis of the crystal structure. Although it is unproven whether phase-pure powder samples can be prepared starting from the composition of the single crystals, this approach allows high-speed identification of new luminescent materials.
3. Green Photonics: Contribution to a Sustainable Lighting Solution
Implementing the ideas of green economics in the field of lighting technology addresses the following three aspects of ecological sustainability: energy consumption, durability, and use of materials.
Compared to conventional lighting, such as incandescent light bulbs and discharge or plasma lighting technology, LED lighting is superior in energy-conversion efficiency. Despite their energy-intensive fabrication, the long service time of several thousand hours (average lifespan of 25,000 hours) of LED lighting systems results in an overall favorable balance. In the future, both a further decrease in energy consumption during service life and a lowered expenditure of energy for fabrication are expected [3].
Here, we want to focus on materials efficiency. We deem this essential especially in view of shortened product and innovation intervals, which increase the risk of obsolescence. The latter is driven by technological progress and by the consumer trend to replace (seemingly) outdated products before the product has reached its end of life.
Several efforts are necessary to increase the ecological sustainability of LED lighting under consideration of the full product lifecycle. Environmentally friendly design and production have to be implemented. So far this goal has been achieved only partially. For example, LED lamps do not require the use of toxic materials, such as mercury. Amongst other features, an ecologically sustainable design will additionally include the development of luminaires with simplified disassembly and recycling.
The use of resources has to be minimized by devising integrated solutions, which could make additional components like drivers obsolete, and by utilizing sustainable (renewable) resources.
Here, we want to present two contributions to more sustainable lighting, namely, (a) recycling and (b) the application of biopolymers for optical components of modern LED lamps.
3.1 Self-Contained Materials Flow
Although recycling is expected to become ecologically more important in the midterm, it is becoming economically more feasible at the same time. Recycling is essential for an ecologically sustainable circular economy and will keep the materials inventory of disposed products available for industrial production by minimizing losses due to waste disposal (e.g., by landfilling or incineration). This is especially true for economically valuable metals or doping agents, the abundance of which is limited by natural deposits.
However, present efforts concentrate on recycling of the more easily retrievable materials of the larger and more homogeneous parts of the lamps such as the casing, socket, or reflector.
The Lightcycle initiative is an example of these efforts. It is a non-profit organization founded in 2005 that is active in Central Europe. It is an approach to enhance the sustainability of lighting systems by tackling the materials cycle of the glass and metal parts of the lamp, which account for the largest portion of volume of end-of-life LED and gas-discharge lamps.
3.2 Biopolymers in Optical Components
In lighting systems, optical materials are subject to several requirements: high transparency in the first place, followed by thermal stability. Additionally, mechanical durability, scratch resistance, and resistivity to chemicals are required depending on the intended application of the lamp.
Several specialized plastics based on fossil resources have been developed in recent years. Generally, these materials are very heterogeneous owing to the need for additivation (e.g., with flame retardants and so forth to meet legal obligations). Thus, recycling is difficult for these materials. The most probable end-of-life option left is combustion.
When these conventional fossil-based raw materials are replaced by bio-based polymers (blends), there is a further end-of-life option for lamps: biodegradation of the bulk material such as housing, lenses, and so forth. Additionally, combustion is an option, which is CO2 neutral in the case of biogenic materials.
A viable solution for the use of biopolymers in the demanding field of optical applications could, therefore, increase the sustainable use of materials in LED-based lighting.
Authors
Jörg Meyer, Frank Tappe, Nico Schmidt,
Hochschule Hamm-Lippstadt, Hamm, Germany
References
[1] European Commission, Frequently asked questions on the regulation phasing out conventional incandescent bulbs, The European Union, http://ec.europa.eu/energy/lumen/doc/full_faq-en.pdf (accessed December 31, 2011).
[2] J. M. Philips, M. E. Coltrin, M. H. Crawford, A. J. Fischer, M. R. Krames, R. Müller-Mach, G. O. Müller, Y. Ono, L. E. S. Rohwer, J. A. Simmons, J. Y. Tsao, Laser Photon. Rev. 2007, 1(4), 307. DOI: 10.1002/lpor.200710019
[3] R. Haitz, J. Y. Tsao, Phys. Status Solidi A 2011, 208(1), 17. DOI: 10.1002/pssa.201026349
[4] M. R. Krames, O. B. Shcheckin, R. Müller-Mach, G. O. Müller, L. Zhou, G. Harbers, M. G. Craford, J. Disp. Technol. 2007, 3, 160.
[5] J. Tsao, M. H. Crawford, M. E. Coltrin, A. J. Fischer, D. D. Koleske, G. S. Subramania, G. T. Wang, J. J. Wierer, R. F. Karlicek, Jr., Adv. Optical Mat. 2014, 2, 809–836. DOI: 10.1002/adom.201400131
[6] G. Blasse, A. Bril, Appl. Phys. Lett. 1967, 11, 53. DOI: 10.1063/1.1755025
[7] H. S. Yoder, M. L. Keith, Am. Mineralog. 1951, 36, 519.
[8] A. A. Setlur, Electrochem. Soc. Interf. 2009, 18, 32. http://www.electrochem.org/dl/interface/wtr/wtr09/wtr09_p032-036.pdf
[9] D. J. Robbins, J. Electrochem. Soc. 1979, 126 (9), 1050. DOI: 10.1149/1.2129328
[10] H. A. Höppe, H. Lutz, P. Morys, W. Schnick, A. Seilmeier, J. Phys. Chem. Solids 2000, 61, 2001. DOI: 10.1016/S0022-3697(00)00194-3
[11] R. Mueller-Mach, G. O. Mueller, M. R. Krames, H. A. Höppe, F. Stadler, W. Schnick, T. Jüstel, P. J. Schmidt, Phys. Status Solidi A 2005, 202(9), 1727. DOI: 10.1002/pssa.200520045
[12] K. Uheda, N. Hirosaki, H. Yamamoto, Phys. Status Solidi A 2006, 203(11), 2712. DOI: 10.1002/pssa.200669576
[13] K. Uheda, N. Hirosaki, Y. Yamamoto, A. Naito, T. Nakajima, H. Yamamoto, Electrochem. Solid State Lett. 2006, 9, H22. DOI: 10.1149/1.2173192
[14] P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt, W. Schnick, Nature Mat. 2014, 13, 891. DOI: 10.1038/nmat4012
[15] J. M. P. J. Verstegen, D. Radielovic, L. E. Vrenken, J. Electrochem. Soc. 1974, 121, 1627. DOI: 10.1149/1.2401757
[16] T. Jüstel, H. Nikol, C. Ronda, Angew. Chem. Int. Ed. 1998, 37, 3084. DOI: 10.1021/cm501866x
[18] J. J. Gallagher, M. a. Hillmyer, T. M. Reineke, ACS Sustain. Chem. Eng. 2015, 662. DOI: 10.1021/sc5008362
Also of interest
- International Year of Light 2015 (IYL 2015)
A global initiative adopted by the United Nations to raise awareness of how optical technologies promote sustainable development and provide solutions to worldwide challenges in energy, education, agriculture, communications, and health.
.jpg)