Desirable characteristics of modern explosives and propellants include high density, positive heats of formation, high detonation velocity and pressure, favorable oxygen balance, high thermal stability, and low sensitivity. Oxygen balance, which indicates the degree to which an explosive can be oxidized, is an important factor in determining the products of decomposition. Traditional methods to achieve a favorable oxygen balance involve introducing nitro groups, as in 2,4,6-trinitrotoluene (TNT), 1,3,5-trinitro-1,3,5-triazinane (RDX), and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX). However, high energy density materials with multiple nitro groups may pose a danger to humans and the environment.
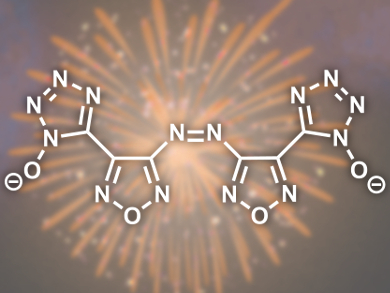
Jean’ne M. Shreeve and co-workers, University of Idaho, Moscow, ID, USA, have addressed this problem by the preparation of two types of salts based on furazan-functionalized tetrazoles (example pictured). As furazan-functionalized tetrazoles are oxygen-rich, they improve the oxygen balance whilst eliminating the need for unnecessary N atoms. Most of the prepared salts possess good detonation properties and low sensitivity to impact and friction. A marked increase in performance was observed relative to the analogous 4-amino-3-(5-tetrazolate)furazan salts and the compounds exhibited excellent thermal stabilities and high positive heats of formation.
The properties of the hydroxylammonium and hydrazinium salts of the developed furazan-functionalized tetrazoles are similar and even superior to that of RDX, which suggests that they may serve as new energetics.
- Energetic Salts Based on Furazan-Functionalized Tetrazoles: Routes to Boost Energy,
Hao Wei, Jiaheng Zhang, Chunlin He, Jean’ne M. Shreeve,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500513