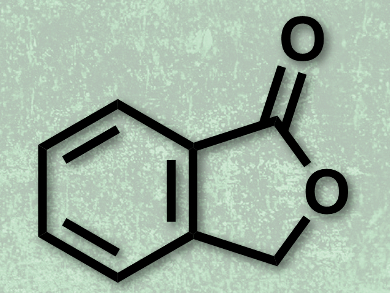
Phthalide (3H-isobenzofuran-1-one, pictured) is an important structural motif found in many natural and bioactive compounds, such as alcyopterosin E, herbaric acid, and sinaspirolide. Several strategies for the synthesis of phthalides by transition metal catalysis have been reported to date. However, these techniques require highly functionalized starting materials and are often limited by substrate availability. Directing group-assisted catalytic C–H activation has emerged as a promising strategy for organic synthesis using relatively unfunctionalized starting materials.
Chien-Hong Cheng and colleagues, National Tsing Hua University, Hsinchu, China, have developed a highly regio- and stereoselective route to 3,3-disubstituted phthalides from aryl carboxylic acids and allenes. The mechanism starts with a rhodium(III)-catalyzed carboxylate-assisted ortho-C–H activation of the carboxylic acid and, therefore, requires less-functionalized starting materials than previously reported syntheses. By using α,β-unsaturated carboxylic acid substrates, this technique can also be used to access highly substituted 2-furanone products.
- Rhodium(III)-Catalyzed [4+1] Annulation of Aromatic and Vinylic Carboxylic Acids with Allenes: An Efficient Method Towards Vinyl-Substituted Phthalides and 2-Furanones,
Parthasarathy Gandeepan, Pachaiyappan Rajamalli, Chien-Hong Cheng,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201501106