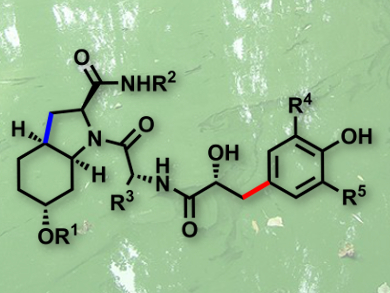
Aeruginosins are marine natural products that have been isolated from sponges and cyanobacterial water blooms. Aeruginosins 298A, 98A–C, and 101 have shown potent in vitro inhibition of various serine proteases, including thrombin and trypsin. These enzymes are involved in a number of important physiological processes, in particular in the blood coagulation cascade. Unfortunately, aeruginosins have only been isolated from dried algae with very low yields. This, combined with their interesting biological properties, encourages an efficient total synthesis of these marine natural products.
Olivier Baudoin and colleagues, Université Claude Bernard Lyon 1, France, have tackled this issue by developing a general and scalable access to the aeruginosin family of marine natural products (example pictured). Their synthesis relies on an intramolecular C–H alkenylation reaction to enable the large-scale synthesis of the common (Choi) heterocyclic core of the target molecules, and an intermolecular directed C–H arylation to furnish diversely decorated hydroxyphenyllactic (Hpla) fragments. The power of this strategy was demonstrated in the synthesis of the four aeruginosins, 298A and 98A–C, two of which, 98A and 98C, have been synthesized for the first time.
Importantly, these syntheses can be scaled up, as demonstrated with aeruginosin 298A, which was obtained in unprecedentedly large quantities (700 mg). This study should facilitate the production of a library of analogues, and allow more advanced pharmacological studies to be carried out.
- Divergent Synthesis of Aeruginosins Based on a C(sp3)-H Activation Strategy,
David Dailler, Grégory Danoun, Benjamin Ourri, Olivier Baudoin,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201501370