Microbial fuel cells (MFCs) have potential applications in many fields, such as wastewater treatment, space shuttles, and marine sediment remediation. MFCs convert organic matter to electricity by electrogenic bacteria growing on the electrode surface. Commercial graphite-based materials, such as carbon felt, carbon cloth, carbon paper, and graphite rods, have been used as anode materials in MFCs. However, the low bacteria loading on these electrodes results in low power output, which hinders practical applications of MFCs. Bacteria are usually unable to access the interior of these electrodes owing to the relatively small pore size.
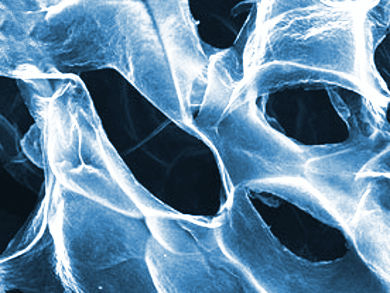
Yueming Tan, Qingji Xie, and co-workers, Hunan Normal University, Changsha, China, have developed a 3D supermacroporous graphene-containing foam (GCF) anode that outperformed carbon cloth in a MFC. The anode was made by mixing agarose with graphite oxide (GO), forming a gel which could be cut into the desired shape and transferred to the substrate. In this case, a stainless-steel mesh was inserted into the GO-agarose gel sample to serve as a current collector in the MFC. Then, the gel was freeze-dried to form the GO-agarose foam, which was pyrolyzed at 350 °C to generate the graphene-containing foam.
The agarose acts as a hardening and crosslinking agent to form the gel and mechanically strengthen the foam, and both graphene and the steel mesh increase the electrical conductivity. Cell viability measurements indicated that the foam possessed excellent biocompatibility. In addition, the supermacroporous structure and high surface area greatly increased the bacterial loading capacity and allowed internal colonization of the anode, which resulted in a maximum area power density of 786 mW m–2, over four times that of a MFC equipped with a commercial carbon cloth anode.
- Facile Fabrication of Graphene-Containing Foam as a High-Performance Anode for Microbial Fuel Cells,
Lu Yang, Shuqin Wang, Shuqin Peng, Hongmei Jiang, Youming Zhang, Wenfang Deng, Yueming Tan, Ming Ma, Qingji Xie,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201501772