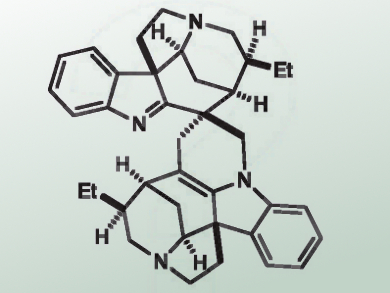
Rodrigo B. Andrade, Temple University, Philadelphia, PA, USA, and colleagues have reported a concise biomimetic syntheses of the Strychnos–Strychnos-type bis-indole alkaloids (–)-leucoridine A and C. Dimerization of (–)-dihydrovalparicine lead to (–)-leucoridine A (pictured). This indolenine could be transformed into (–)-leucoridine C by ring-opening using hydrochloric acid.
The researchers prepared (–)-dehydrogeissoschizoline starting from commercially available N-tosyl indole 3-carboxaldehyde via (–)-dihydroakuammicine and (+)-geissoschizoline. Dehydration of the product with with trifluoroacetic acid (TFA) in the presence of 4 Å molecular sieves gave (–)-dihydrovalparicine, which dimerized in situ to afford (–)-leucoridine A.
DFT calculations were used to understand the mechanism of the dimerization. The results make a stepwise aza-Michael/spirocyclization sequence seem more likely than the alternate hetero-Diels-Alder cycloaddition reaction. For the concerted mechanism, no viable transition state could be found.
- Biomimetic Total Syntheses of (–)-Leucoridines A and C through the Dimerization of (–)-Dihydrovalparicine,
Praveen Kokkonda, Keaon R. Brown, Trevor J. Seguin, Steven E. Wheeler, Shivaiah Vaddypally, Michael J. Zdilla, Rodrigo B. Andrrade,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201505198