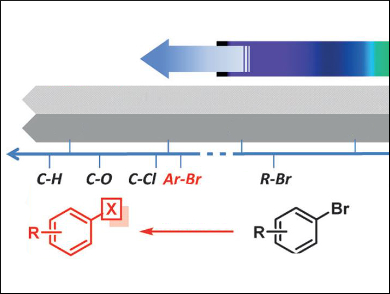
Visible light provides an abundant source of clean energy, but its use in organic synthesis has long been limited to the cleavage of weaker bonds, such as C–I or C(sp3)–Br, owing to the low energy of single photons. Dissociation of aryl-Br bonds was hitherto off-limits to visible light, as the bond energies are too high.
This limitation has been overcome by Axel Jacobi von Wangelin and colleagues, University of Regensburg, Germany, by using photon upconversion by triplet-triplet annihilation (TTA). This is its first reported use in bond activation. TTA involves energy transfer between a sensitizer (in this case butane-2,3-dione, BD) and an acceptor or annihilator (here 2,5-diphenyloxazole, PPO), which allows the energies of two long-lived excited triplet states to be combined.
The group applied this approach to the reduction of various aryl bromides. The process exhibits a Vis-to-UV photon upconversion upon excitation by laser flash photolysis. This effects single electron transfer-initiated reductive activation of the Ar–Br bond to form the aryl radical and Br–. The reaction proceeds under mild reaction conditions with low-energy visible light.
- Application of Visible-to-UV Photon Upconversion to Photoredox Catalysis: The Activation of Aryl Bromides,
Michal Majek, Uwe Faltermeier, Bernhard Dick, Raúl Pérez-Ruiz, Axel Jacobi von Wangelin,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502698
Also of Interest
- International Year of Light 2015 (IYL 2015)
A global initiative adopted by the United Nations to raise awareness of how optical technologies promote sustainable development and provide solutions to worldwide challenges in energy, education, agriculture, communications, and health.

![Diazine-Tetraphenylethylene Cyclo[6]arenes for Molecular Recognition in Solution and Aggregate States](https://www.chemistryviews.org/wp-content/uploads/2025/11/ChemistryViews-2.70171-125x94.png)
