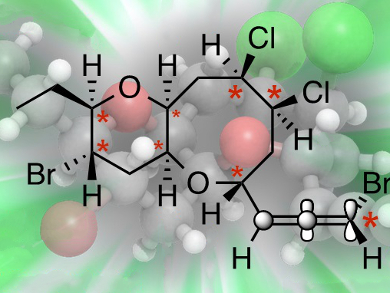
Microcladallenes A, B, and C are natural products that have previously been isolated from the red algae Laurencia microcladia. Proposed structures of compounds A and C have been suggested based on proton and carbon NMR spectroscopy, whereas an absolute configuration of compound B has been confirmed using X-ray crystallography.
Deukjoon Kim, Seoul National University, Republic of Korea, and colleagues have accomplished the substrate-controlled asymmetric total syntheses of (+)-microcladallenes A, B, and C based on the proposed structures. The syntheses of A and B confirmed the structures and absolute configurations of both natural products. However, the synthesis of C, which includes seven stereogenic centers and an (R)-bromoallene in its compact C15 framework, revealed that its proposed structure must be revised.
X-ray crystallographic analysis coupled with chemical transformations strongly suggested that the originally proposed structure of C was incorrect, so an unambiguous structure assignment needs a future reisolation and analysis of the natural product.
- Substrate-Controlled Asymmetric Total Syntheses of Microcladallenes A, B, and C Based on the Proposed Structures,
Te-ik Sohn, Deukjoon Kim, Robert S. Paton,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502592