Supramolecular interactions, such as H-bonding or π-stacking, can be exploited to tune catalytic reactions, usually by altering the reaction selectivity. For example, π-stacking between the ligands of catalysts and the substrate can effect asymmetric induction. However, noncovalent interactions can also affect the rates of catalytic reactions, as reported by Sheila Ruiz-Botella and Eduardo Peris of Universitat Jaume I, Castellón, Spain.
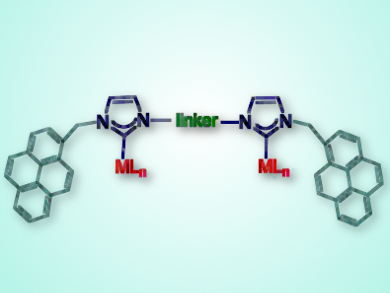
The team tested an iridium-NHC complex decorated with rigid aromatic pyrene groups (pictured) as a homogeneous catalyst for reduction of ketones by transfer hydrogenation and β-alkylation of secondary alcohols – two reactions proceeding by the borrowing hydrogen methodology. If aromatic substrates are used, the addition of π-stacking additives inhibited the reactions.
In the absence of additives, reactions with aromatic substrates follow zero-order dependence on substrate concentration, presumably owing to saturation of the catalyst by π-stacking with the substrate, whereas, with aliphatic substrates, the reaction exhibits second-order substrate dependence. The reactions follow a reaction order of <1 in the pyrene-containing catalyst, indicating that the catalyst self-associates. The pyrene tags were also used to immobilize the catalyst on a reduced graphene oxide surface, giving a recyclable catalyst for β-alkylation of secondary alcohols.
- Unveiling the Importance of π-Stacking in Borrowing-Hydrogen Processes Catalysed by Iridium Complexes with Pyrene Tags,
Sheila Ruiz-Botella, Eduardo Peris,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502948



“Supramolecular interactions, such as H-bonding or π-stacking, can be exploited to tune catalytic reactions, usually by altering the reaction selectivity. For example, π-stacking between the ligands of catalysts and the substrate can effect asymmetric induction.”
Hi David, you have clearly written what can be done for supramolecular interactions. As far as I know, this is not so clear at all so far. And I am going to propose in this direction for my postdoc. Can you send me the specific examples or post it here?