The design and fabrication of oxygen reduction reaction (ORR) electrocatalysts with high performance at low cost remains a big challenge, but is crucial for the commercialization of fuel cells. Platinum remains the state-of-the-art catalyst for the ORR, despite its prohibitive cost, limited resources, and degradation, which impede the large-scale commercialization of these sustainable technologies.
Heteroatom-doped graphenes (HDGs) have emerged as promising ORR electrocatalysts owing to their low cost, good tolerance toward fuel, and durability. Unfortunately, the two approaches commonly used to prepare HDGs require either high-temperature annealing and hydrothermal treatment of graphene oxide with multiple heteroatom-containing precursors, which are generally expensive and toxic, or the use of hazardous oxidants and tedious multistep manipulations. In addition, the ORR activities of these graphenes are still inferior to those of commercial Pt/C catalysts.
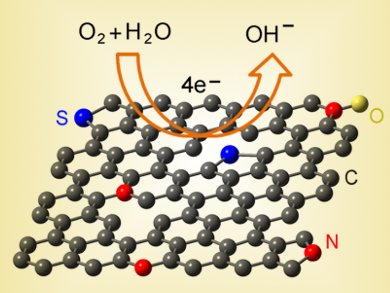
Junyan Zhang and colleagues, Lanzhou Institute of Chemical Physics, China, have developed a facile and economical method for the fabrication of N and S dual-doped graphene (NS-G) nanosheets by pyrolysis of cysteine in the presence of NaCl. Cysteine can be produced industrially by the hydrolysis of cheap poultry feathers or hair. In this strategy, cysteine is used as the sole source of nitrogen, sulfur, and carbon. It covers the surface of self-assembled NaCl particles, which then act as a structure-directing template to direct the growth of graphene. Following removal of the NaCl template and a second heating treatment, the resultant NS-G possesses uniform N/S co-doping with 1.85 at% N and 0.99 at% S, along with a high surface area (435.13 m2/g), mesoporous structure, and improved electrical conductivity.
The dopes graphene exhibits excellent activity toward the ORR, comparable with commercial Pt/C catalyst, coupled with high electrochemical stability in alkaline medium. Together with the convenient preparation, low-cost raw materials, and high performance, this material might be a promising cathodic oxygen reduction reaction electrocatalyst for fuel cells.
- A Facile Synthesis of Nitrogen/Sulfur Co-Doped Graphene for the Oxygen Reduction Reaction,
F. Pan, Y. Duan, X. Zhang, J. Zhang,
ChemCatChem 2015.
DOI: 10.1002/cctc.201500893