Carbohydrates are tricky candidates for chemical synthesis. Their many hydroxyl groups, each of which has its specific stereochemistry, can make targeted functionalization difficult. Despite this, Chao-Jun Li, McGill University, Montreal, Canada, and colleagues have developed a mild one-pot synthesis for the olefination of monosaccharides. This reaction combines hydrazine and palladium chemistry to form new carbon–carbon bonds and add alkene functionality to sugars.
Attaching Allyl or 1,3-Diene Groups
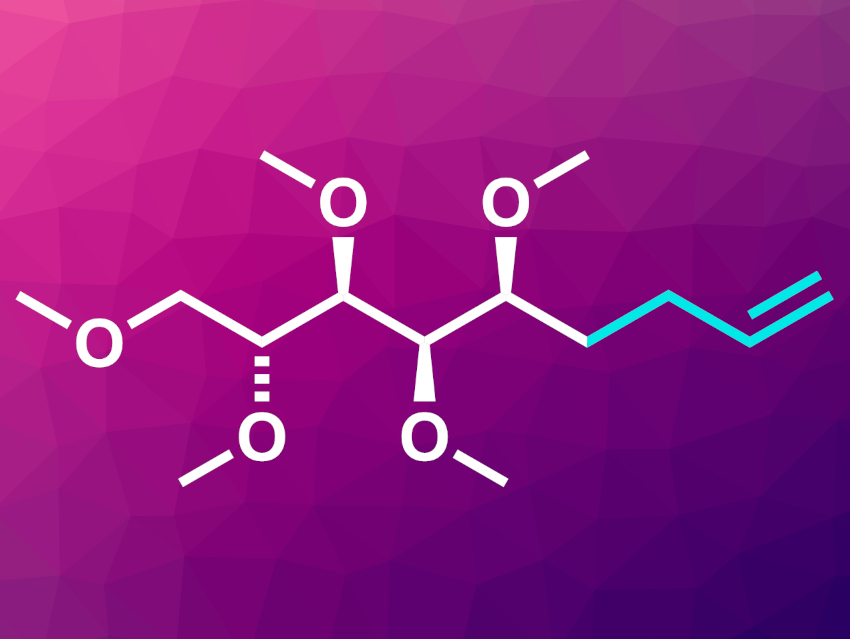
The team focused on the carbonyl group of monosaccharides. By reversing the polarity of the carbonyl carbon, a step called umpolung, the team was able to attach an electrophilic allyl group to it, while it was simultaneously deoxygenated (example product pictured). In a variation of their synthesis, they dehydrogenated the allyl product further to form a 1,3-diene.
Adding an allyl group produces two assets to synthesis: one is the direct formation of a carbon–carbon bond, always desirable in synthetic chemistry. The other is the generation of a terminal double bond, a highly useful functionality for further transformations.
To explore the possibilities of the newly generated olefin products, the researchers synthesized derivatives of a stereochemically demanding natural product—the anticarcinogenic compound anamarine—in two steps. They first deoxygenatively allylated a sugar substrate (D-ribose, L-arabinose, or L-fructose) to form the terminal alkene. Afterward, the product was transformed into the anamarine derivative in an olefin metathesis reaction.
For the deoxygenative allylation, the team first created the umpolung reagent by simply adding hydrazine monohydrate to the sugar, giving a hydrazone. Then, a palladium catalyst was used to activate allyl acetate, which reacted with the hydrazone to form the allyl product in the presence of a strong base. Another catalyst, made in situ from palladium allyl chloride and a phosphine ligand, led to the 1,3-diene product.
Combining Three Well-Known Reactions
With this procedure, the researchers succeeded in combining three well-known transformations, all of which are well established in organic chemistry. The first is known from sugar analytics and is the use of a hydrazine reagent to detect monosaccharides: an excess of phenylhydrazine converts reducing sugars to osazones, which precipitate from the acidic solution as yellow crystals.
The second transformation is the Wolff-Kishner reduction, which was established in the early 1920s. It also uses hydrazine, but proceeds under alkaline conditions, where carbonyl groups become fully deoxygenated. The third reaction used by the team is a palladium-catalyzed allylation, which was developed in the 1960s. Here, palladium catalysts transition between the oxidation states zero and two to add allyl groups to electron-rich carbon atoms.
Taken together—hydrazine chemistry on sugars, alkaline conditions, and palladium-catalyzed allylation, this reaction works in one pot and at room temperature or with mild heating. To demonstrate the scope of this method, the team transformed an impressive number of monosaccharide derivatives to their olefin products.
Nevertheless, all reactions were run on the NMR scale, so there is still work to do to achieve a broader synthetic utility. And although the researchers claim that free sugars reacted well, they also admit that the variants where the hydroxyl groups were protected gave better yields. This means that even with this type of functionalization, protecting group chemistry continues to play a crucial role.
- Umpolung carbonyls enable direct allylation and olefination of carbohydrates,
Jian Kan, Zhangpei Chen, Zihang Qiu, Leiyang Lv, Chenchen Li, Chao-Jun Li,
Sci. Adv. 2022.
https://doi.org/10.1126/sciadv.abm6840