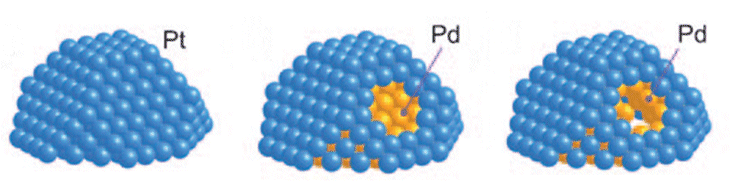
In current nanoparticular fuel-cell cathodes systems, platinum nanoparticles (NPs) can dissolve and redeposit on other PtNPs, lowering their effectiveness.
Working with colleagues from the Brookhaven National Laboratory and the Toyota Motor Corporation, Radoslav Adzic and co-workers have shown that platinum monolayers can act as shells for palladium NPs. This led to electrocatalysts with high activities and an ultralow platinum content, but high platinum utilization (see surface models of the NPs).
The palladium core increases the stability of a platinum monolayer shell by positively shifting the platinum oxidation potential and impeding the platinum dissolution by slow dissolution of palladium. In fuel-cell tests, no loss of platinum was observed in 200,000 potential cycles, whereas loss of palladium was significant.
- Core-Protected Platinum Monolayer Shell High-Stability Electrocatalysts for Fuel-Cell Cathodes
K. Sasaki, H. Naohara, Y. Cai, Y. M. Choi, P. Liu, M. B. Vukmirovic, J. X. Wang, R. R. Adzic,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201004287 - K. Sasaki, H. Naohara, Y. Cai, Y. M. Choi, P. Liu, M. B. Vukmirovic, J. X. Wang, R. R. Adzic,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201004287



