New natural product analogues with potential applications in medicinal chemistry have been prepared by using unusual amino sugar building blocks.
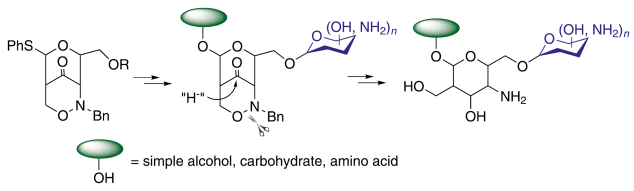
Hans-Ulrich Reissig and Fabian Pfrengle, Freie Universität Berlin, Germany, have utilized the Lewis acid mediated rearrangement of phenylthio-substituted 1,2-oxazines to deliver glycosyl donor equivalents that can directly be employed in glycosidation reactions.
Deprotection of the amino sugar precursors by simple reductive steps provides new natural product analogues having C2-branched 4-amino sugar units with different absolute and relative configurations.
The use of thiophenyl-substituted 1,2-oxazine derivatives now allows not only the synthesis of mimetics but of “real” carbohydrates bearing an anomeric center.
- Internally Protected Amino Sugar Equivalents from Enantiopure 1,2-Oxazines: Synthesis of Variably Configured Carbohydrates with C-Branched Amino Sugar Units
F. Pfrengle, H.-U. Reissig,
Chem. Eur. J. 2010, 16(39).
DOI: 10.1002/chem.201001060




