Isolation studies targeting Croton plants have yielded a wealth of structurally intriguing and biologically active natural products. Recent efforts aimed at identifying potentially medicinal diterpenoid compounds extracted from the leaves of Croton Steenkampianus resulted in the isolation of two new compounds with moderate antiplasmodial activities, which were named (+)-steenkrotins A and B.
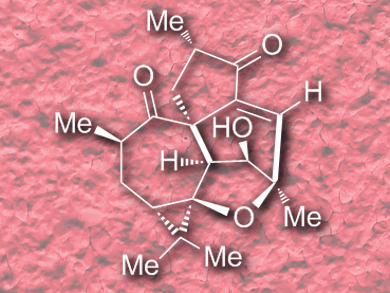
Hanfeng Ding and co-workers, Zhejiang University, Hangzhou, China, have accomplished the first enantioselective total synthesis of (+)-steenkrotin A (pictured) in 18 steps and 4.2 % overall yield, starting from an easily accessible chiral cycloheptenone. The strategy includes three key ring formations: 1) a Rh-catalyzed O–H bond insertion followed by an intramolecular carbonyl-ene reaction to construct the tetrahydrofuran subunit; 2) two sequential SmI2-mediated Ueno-Stork and ketyl-olefin cyclizations to build up the [5,7]spirobicyclic skeleton; and 3) a cascade intramolecular aldol condensation/vinylogous retro-aldol/aldol reaction to forge the final six-membered ring with inversion of the relative configuration at the C7 position.
The described synthesis established the absolute configuration of (+)-steenkrotin A by stepwise construction of the stereocenters. The team hopes that the methodologies and strategies employed will find further application in the synthesis of other complex natural products.
- Enantioselective Total Synthesis of (+)-Steenkrotin A and Determination of Its Absolute Configuration,
Saiyong Pan, Beiling Gao, Jialei Hu, Jun Xuan, Hujun Xie, Hanfeng Ding,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201503831