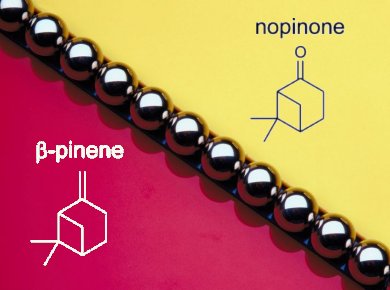
The use of mechanical energy as input to perform chemical reactions is becoming more common in chemical research as it removes the need for, and removal of, solvents.
Achim Stolle and colleagues, Friedrich-Schiller University Jena, Germany, report a solvent-free method for the synthesis of nopinone from the renewable monoterpene β-pinene by using a ball mill and an auxiliary grinding agent.
They show that it is possible to cleave β-pinene oxidatively into nopinone with KMnO4 as the oxidant. Compared with classical ozonolysis, the solvent-free conditions avoided long reaction times (hours compared to several minutes), the use of solvents, and the generation before reaction and work-up of O3 and ozonides after the reaction.
The best results were achieved by co-grinding 2 mmol β-pinene with 6 mmol KMnO4 and 3.8 g of Al2O3, for 10 min to achieve high yields of nopinone (95 %).
- An Alternative Solvent-Free Synthesis of Nopinone under Ball-Milling Conditions: Investigation of Reaction Parameters
T. Szuppa, A. Stolle, B. Ondruschka, W. Hopfe,
ChemSusChem 2010, 3 (10), 1089–1207.
DOI: 10.1002/cssc.201000122