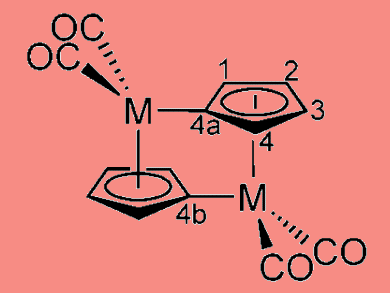
Researchers around Jeffrey Grossman at MIT have revealed how fulvalene diruthenium works to store and release heat on demand. This understanding should make it possible to find similar chemicals based on more abundant, less expensive materials than ruthenium, and this could form the basis of a rechargeable battery to store heat rather than electricity.
Fulvalene diruthenium undergoes a structural transformation when it absorbs sunlight, putting it into a higher-energy state where it can remain stable indefinitely. Then, triggered by a small addition of heat or a catalyst, it snaps back to its original shape, releasing heat. An intermediate step plays a major role here: The molecule forms a semi-stable configuration partway between the two previously known states. The two-step process helps explain why the molecule is so stable, why the process is easily reversible and why substituting other elements for ruthenium has not worked so far.
.gif)
A relatively rapid preequilibrium between cyclopentadienyl complex 2 and fulvalene diradical complex 1 precedes the rate-determining anti–syn rotation and formation of the Ru Ru bond.
Grossman plans to collaborate with Daniel Nocera, MIT, to apply the principles learned from this analysis in order to design new, inexpensive materials that exhibit this same reversible process.
- Mechanism of Thermal Reversal of the (Fulvalene)tetracarbonyldiruthenium Photoisomerization: Toward Molecular Solar–Thermal Energy Storage
Yosuke Kanai, Varadharajan Srinivasan, Steven K. Meier, K. Peter C. Vollhardt, Jeffrey C. Grossman
Angew. Chem. Int. Ed. 2010
DOI: 10.1002/anie.201002994 - Mechanism of Thermal Reversal of the (Fulvalene)tetracarbonyldiruthenium Photoisomerization: Toward Molecular Solar–Thermal Energy Storage,
Yosuke Kanai, Varadharajan Srinivasan, Steven K. Meier, K. Peter C. Vollhardt, Jeffrey C. Grossman
Angew. Chem. 2010.
DOI: 10.1002/ange.201002994
- Video: Solar Energy for the Poor?, Daniel Nocera, MIT, USA