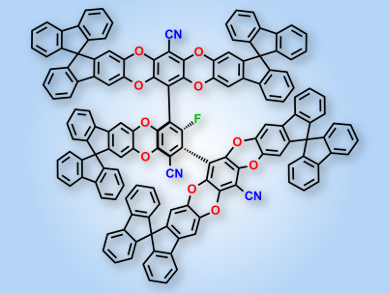
The reaction between fluorinated biphenyl and terphenyl cores with catechol-functionalized terminal groups results in molecular adducts with well-defined concavities. These organic molecules of intrinsic microporosity (OMIMs) provide porous solids solely by their inefficient packing.
Neil B. McKeown and his colleagues, University of Edinburgh, Scotland, UK, engaged in a joint modeling and synthesis program to investigate the microporosity generated by these compounds. By altering the number, size, and bulk of the terminal groups, a number of OMIMs with surface areas of more than 600 m2g–1 were produced.
The introduction of bulky hydrocarbon substituents significantly enhances microporosity by further reducing packing efficiency. The introduction of methyl groups at the bridgehead position of the triptycene units reduces the intrinsic microporosity. This is presumably a result of their internal position within the OMIM structure: they occupy space, but unlike peripheral substituents, they do not contribute to the generation of free volume by inefficient packing.
- The Synthesis of Organic Molecules of Intrinsic Microporosity Designed to Frustrate Efficient Molecular Packing,
Rupert G. D. Taylor, C. Grazia Bezzu, Mariolino Carta, Kadhum J. Msayib, Jonathan Walker, Rhys Short, Benson M. Kariuki, Neil B. McKeown,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201504212